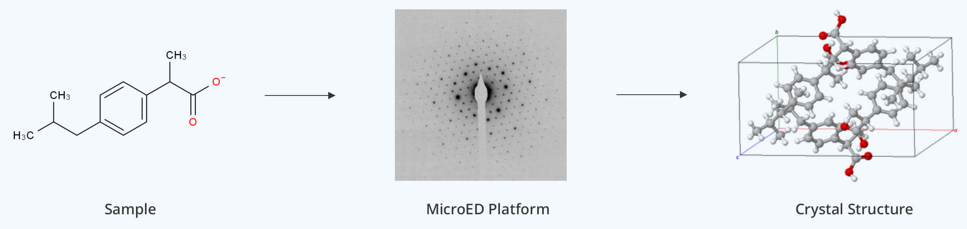
What is Microcrystal Electron Diffraction (MicroED)?
Microcrystal Electron Diffraction (MicroED) is an innovative structural analysis method that uses electron diffraction patterns to reveal the atomic structure of crystals as tiny as a few nanometers. By integrating cryo-electron microscopy (cryo-EM) with electron diffraction, MicroED facilitates high-resolution 3D imaging of micro-sized crystals. Unlike traditional X-ray crystallography, which requires large, high-quality crystals, MicroED can analyze much smaller and more complex samples.
This advancement overcomes the limitations of conventional X-ray diffraction in structural biology, making it particularly valuable for studying intricate macromolecules like membrane proteins (such as GPCRs) and small-molecule compounds. MicroED is opening up new avenues for drug discovery, materials science, and the examination of biomolecules that were previously too challenging to study. In 2018, Science magazine recognized MicroED as one of the top ten technological breakthroughs of the year.
Principle of MicroED Technology
The core of MicroED lies in the intriguing combination of electron wave-particle duality and Bragg's diffraction law. When an electron beam interacts with a crystalline material, it generates a series of diffraction patterns due to the interference of scattered electron waves. These patterns, captured by a detector, reveal crucial information about the crystal's atomic structure and lattice arrangement.
Bragg's law governs this phenomenon: as the electron beam strikes the crystal, it produces diffraction spots that correspond to the crystal's periodic atomic arrangement. The position and intensity of these spots are determined by the crystal's atomic structure, lattice parameters, and the specific angle and wavelength of the electron beam.
In MicroED, the electron beam is precisely focused on a tiny region of the sample, often at the nanoscale, using a transmission electron microscope (TEM). By varying the angle of the electron beam, different diffraction patterns are captured, providing detailed insights into the crystal's structure. This technique enables high-resolution analysis, even for micro-sized crystals, overcoming the limitations of traditional X-ray diffraction methods and making it a crucial tool for studying even the most challenging samples.
Development History of MicroED Technology
MicroED technology has evolved through several key stages, from early electron crystallography to its current role in structural biology and material science.
1. Early Electron Crystallography (1970s–2000s)
Foundation of Electron Crystallography: In 1975, Aaron Klug laid the theoretical foundation for electron crystallography by extracting phase information from electron micrographs using Fourier transforms. The Richard Henderson team solved the structure of bacterial rhodopsin at 7 Å resolution in 1975, demonstrating the potential of electron crystallography for studying membrane proteins.
Limitations of 2D Crystals: By 2005, the structure of aquaporin AQP0 was solved at 1.9 Å resolution, marking the maturity of electron crystallography. However, the growth of 2D crystals proved difficult, limiting their broader application.
2. Emergence of MicroED (2013)
Proof of Concept: In 2013, Gonen's team introduced MicroED by successfully solving the structure of lysozyme microcrystals at 2.9 Å resolution using cryo-electron microscopy. This demonstrated the feasibility of 3D microcrystal electron diffraction. The team utilized continuous tilting and low-dose cryogenic techniques (<10 e⁻/Ų) to prevent radiation damage.
3. Technological Breakthroughs and Biological Applications (2016–2018)
Direct Phase Determination: In 2016, Eisenberg's team solved the structure of prion protein nanocrystals at 1.4 Å resolution using direct methods for phase determination, a first in MicroED. This overcame previous limitations due to dynamic scattering.
Automation and Sample Preparation:
- Continuous Rotation Electron Diffraction (cRED): 2018 saw the development of automated data collection software like Instamatic, improving efficiency and reducing human error.
- Focused Ion Beam (FIB) Milling: This technique was applied to lipid cubic phase (LCP) gel samples, thinning microcrystals to 100-300 nm, thereby minimizing multiple scattering interference.
4. High-Resolution Breakthroughs and Expanding Applications (2019–2020)
Atomic Resolution Breakthrough: In 2019, MicroED resolved the toxic core of α-synuclein (1.4 Å) and the human adenosine A2A receptor (2.8 Å), and observed the cholesterol molecule binding site in GPCR for the first time.
Small Molecules and Drug Development: Jones et al. demonstrated MicroED's atomic resolution (<1 Å) structural analysis capability for small organic molecules (such as drug precursors), requiring only nanogram samples and no single crystals.
How Does MicroED Work?
1. Sample Preparation for MicroED
The preparation of samples for MicroED involves growing crystals in a LCP or solution, followed by vitrification to prevent ice formation during electron microscopy. In some cases, the sample may be thinned to 100-300 nm using a FIB to reduce electron scattering. This step is crucial to achieving high-quality diffraction patterns from even the smallest crystals.
2. Data Collection in MicroED
Once the sample is prepared, it is analyzed in a TEM. In this process, the sample is continuously tilted, typically by 0.1 to 1 degree, to collect multiple diffraction images from different orientations. New detectors, such as the Falcon III, have significantly enhanced the efficiency of data acquisition by reducing radiation damage while improving the overall speed of the process.
3. Data Processing and Structure Solution in MicroED
The diffraction data is processed using specialized crystallography software such as PHENIX and Scipion-ED. The phase problem, which is common in crystallography, is often solved using molecular replacement or direct methods. The result is a high-resolution 3D structure, which can achieve resolution as fine as 1.7 Å or even higher, depending on the sample and experimental conditions.
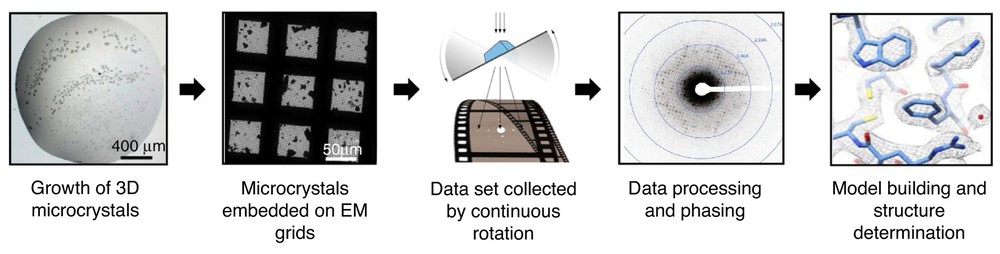 Figure 1. MicroED Workflow for Structure Determination. The MicroED process involves crystal growth, screening, electron diffraction collection, and structure refinement through crystallography software. (Nguyen C, et al., 2020)
Figure 1. MicroED Workflow for Structure Determination. The MicroED process involves crystal growth, screening, electron diffraction collection, and structure refinement through crystallography software. (Nguyen C, et al., 2020)
MicroED Technology Applications
1. Protein Crystal Structure Determination
MicroED technology enables the high-resolution 3D structure analysis of protein crystals. Unlike traditional X-ray crystallography, MicroED can work with small crystals, reducing the need for large, high-quality crystals. This approach not only makes it possible to analyze proteins that are difficult to crystallize but also allows for higher resolution data. For example, R2lox enzyme was independently solved for its structure using MicroED, demonstrating its potential in studying unknown biological molecules.
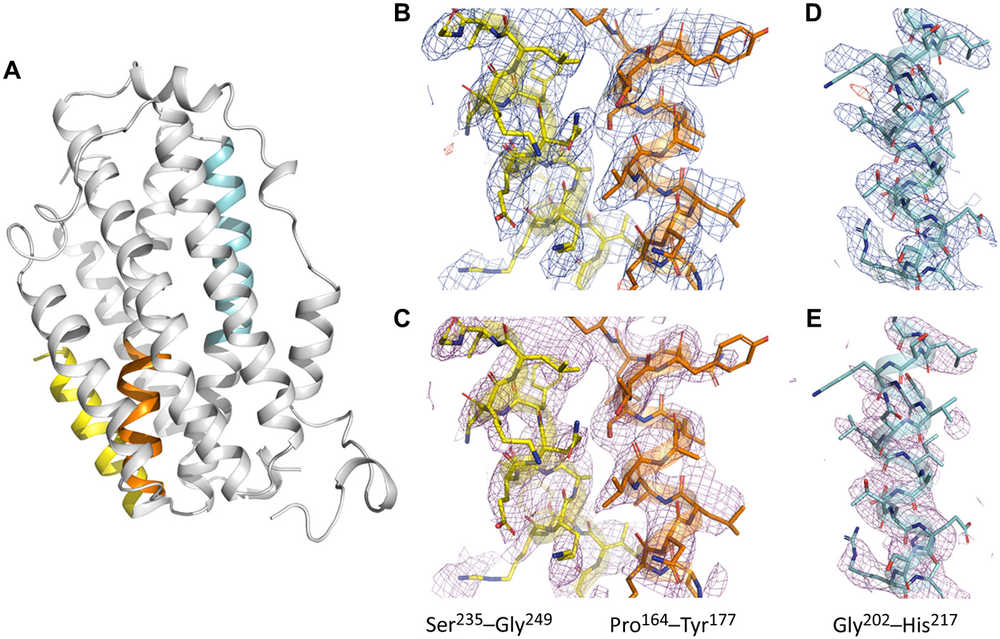 Figure 2. MicroED Application for High-Resolution Electrostatic Mapping of SaR2lox. (Xu H, et al., 2019)
Figure 2. MicroED Application for High-Resolution Electrostatic Mapping of SaR2lox. (Xu H, et al., 2019)
2. Structural Analysis of Biomolecules
Beyond proteins, MicroED is also highly effective in resolving the structures of biomolecules such as DNA, RNA, carbohydrates, and other complex biological macromolecules. These molecules often present challenges for traditional X-ray crystallography, but MicroED can successfully provide high-resolution 3D structural information. This expands its application to areas where other techniques fall short. For example, MicroED reveals the 1.10 Å resolution structure of d(CGCGCG)2 DNA, showcasing the potential of cryo-FIB milling for nucleic acid crystals.
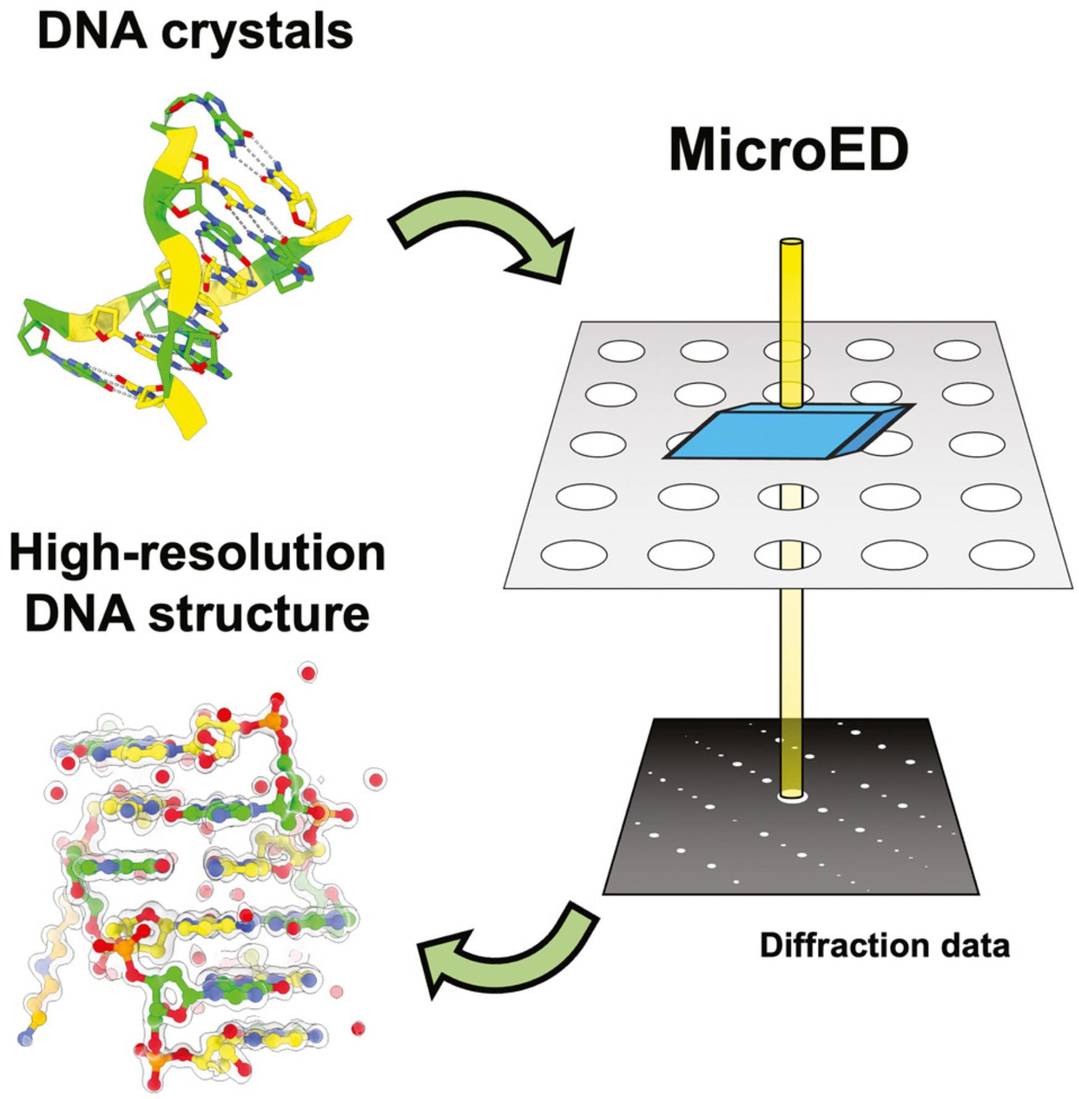 Figure 3. High-Resolution DNA Structure Determination Using MicroED. (Haymaker A, et al., 2023)
Figure 3. High-Resolution DNA Structure Determination Using MicroED. (Haymaker A, et al., 2023)
3. MicroED in Drug Research
Protein-Ligand Interactions
MicroED plays a crucial role in drug research, particularly in studying the interactions between drugs and proteins. In drug discovery, the binding of drug molecules to protein targets often occurs in amorphous or microcrystalline states, conditions where traditional X-ray crystallography cannot be applied. MicroED provides high-resolution structural insights into these interactions, which is essential for understanding the mechanisms of drug binding. For instance, MicroED has been used to analyze GPCRs such as the human adenosine A2A receptor at 2.8 Å resolution, revealing ligand binding sites and interactions with cholesterol molecules.
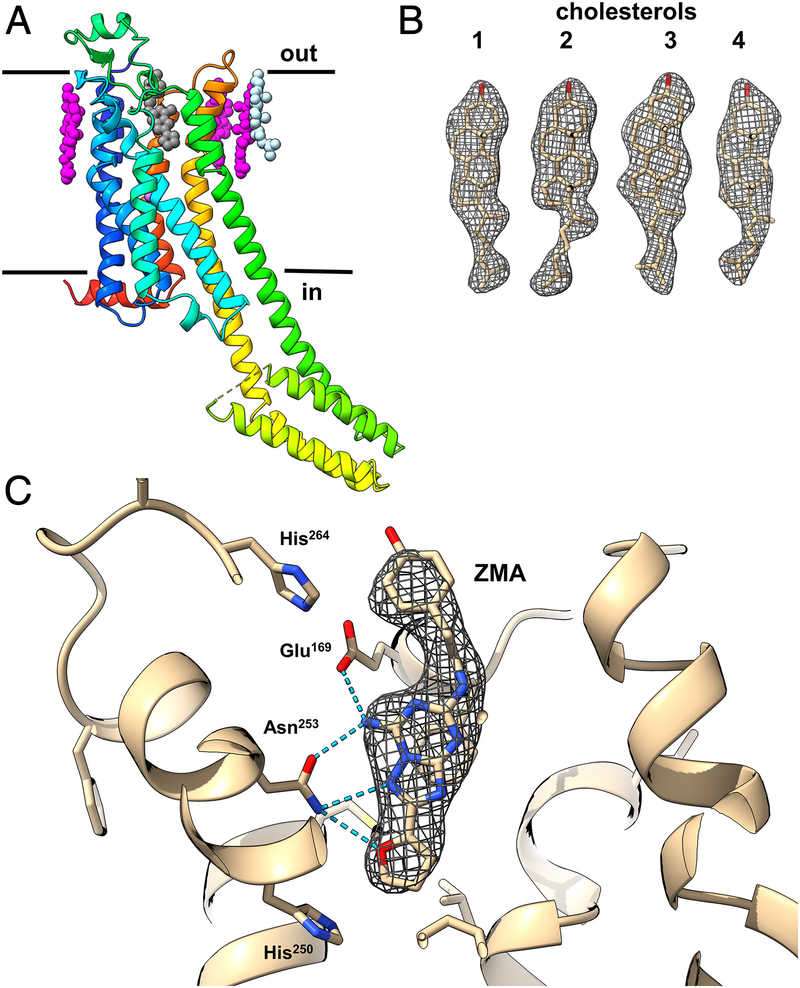 Figure 4. MicroED Structural Insights into A2AAR GPCR. MicroED reveals the structure of A2AAR GPCR, highlighting ligand binding sites and cholesterol interactions for better drug targeting. (Martynowycz M W, et al., 2021)
Figure 4. MicroED Structural Insights into A2AAR GPCR. MicroED reveals the structure of A2AAR GPCR, highlighting ligand binding sites and cholesterol interactions for better drug targeting. (Martynowycz M W, et al., 2021)
Small Molecule Compound Structure Determination
MicroED has become a powerful tool for determining the structure of small molecules, including organic and inorganic compounds, peptides, and natural products. It can analyze small crystals or even powders, eliminating the need for fully grown crystals. For example, MicroED was used to resolve the structure of carbamazepine, a drug for epilepsy and bipolar disorder, by identifying hydrogen atom positions.
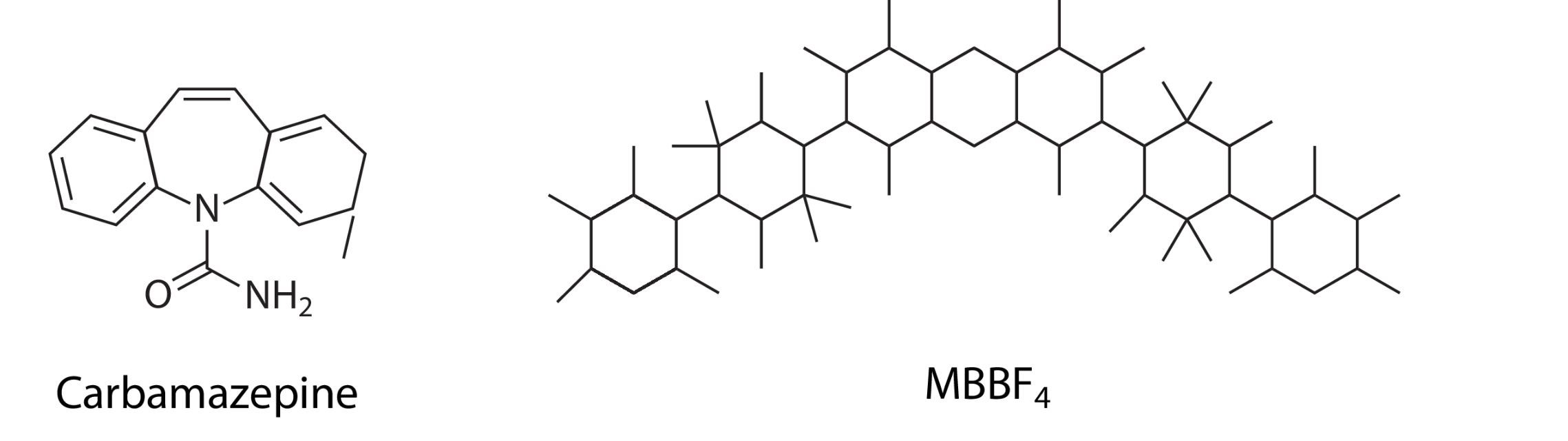 Figure 5. Small-Molecule Structures Solved by MicroED. Examples of small-molecule structures determined using MicroED, including carbamazepine and MBBF4. (Mu X, et al., 2021)
Figure 5. Small-Molecule Structures Solved by MicroED. Examples of small-molecule structures determined using MicroED, including carbamazepine and MBBF4. (Mu X, et al., 2021)
4. MicroED in Materials Research
In the field of materials science, MicroED is also invaluable for determining the structures of framework compounds and inorganic materials such as MOFs, COFs, HOFs, zeolites, and other organic small molecules and metal oxides. These materials often pose significant challenges in forming large, high-quality crystals suitable for traditional X-ray crystallography. However, MicroED enables the high-resolution structural characterization of these materials, providing critical insights into their structural properties and behavior.
For instance, it was used to determine the structure of the conductive MOF Cu₃(C₆O₆)₂ with sub-angstrom precision, revealing unique π-stacking interactions and extremely short (2.73 Å) ligand packing caused by pancake bonding. This discovery provided insights into the material's semiconductive properties, attributed to the localized nature of the pancake bonds and the formation of a singlet dimer. Additionally, MicroED uncovered the role of Cu²⁺ spin in the material's magnetic behavior, highlighting strong geometrical magnetic frustration within its Kagomé arrangement. Such capabilities make MicroED a crucial tool for advancing our understanding of material properties at the atomic level.
 Figure 6. Structural Insights into Cu₃(C₆O₆)₂ MOF via MicroED. (Meng Z, et al., 2022)
Figure 6. Structural Insights into Cu₃(C₆O₆)₂ MOF via MicroED. (Meng Z, et al., 2022)
Advantages of MicroED Technology
- Tiny Sample Requirements: One of the key advantages of MicroED is its ability to work with exceptionally small crystals, down to the nanometer scale. This eliminates the need for large, high-quality crystals that are typically required for X-ray diffraction, making it possible to study a wider range of samples, including rare or difficult-to-crystallize molecules.
- High Resolution: MicroED offers an unprecedented level of resolution, with the ability to resolve structures at the atomic level, even identifying the positions of hydrogen atoms. This capability enables a deeper understanding of molecular structures, which is crucial for both drug development and materials science.
- Versatility Across Different Sample Types: MicroED is highly versatile, capable of analyzing small molecules, peptides, membrane proteins, and materials science samples. This flexibility makes it an invaluable tool in many fields, from drug discovery to materials optimization.
- Low-Temperature Protection with Cryo-EM: The use of cryo-EM in MicroED helps preserve the sample's natural conformation by preventing radiation damage through vitrification. This is particularly important for biological samples, which can easily lose their native structures under standard electron microscopy conditions.
Challenges and Limitations of MicroED Technology
- Radiation Damage: While MicroED provides high-resolution data, it does come with challenges related to radiation damage. The electron beam used in the technique can cause local damage to the sample, including displacement of metal ions or breaking of disulfide bonds. Researchers are continuously working on optimizing the dose control to minimize this effect.
- Sample Preparation Challenges: Sample preparation for MicroED can be complex, particularly when working with LCP. The crystals often require additional processing steps to improve diffraction quality, such as converting them into sponge phases or enzymatically cleaving them.
- Phase Problem: Like all diffraction techniques, MicroED faces the phase problem, which makes it difficult to directly obtain structural information from diffraction patterns. This issue is typically resolved using molecular replacement or by combining MicroED with other techniques, such as cryo-EM single-particle analysis.
- Equipment Dependency: The advanced technology required for MicroED, such as high-performance detectors and FIB systems, is expensive, which can limit the accessibility of the technique. However, as the technology continues to develop and costs decrease, MicroED will likely become more widely available.
Recent Advancements in MicroED Technology
Innovations in Detectors and Automation
Recent advancements in detector technology, such as the Falcon III and CMOS detectors, have improved the efficiency and speed of data acquisition while reducing the radiation dose required for high-quality diffraction patterns. Additionally, software like Scipion-ED has automated the data processing pipeline, significantly enhancing throughput.
Sample Preparation Innovations for MicroED
New innovations in sample preparation, such as 3D-printed suspended drop crystallization devices, have helped address issues like crystal orientation, making it easier to prepare samples for MicroED analysis.
Absolute Configuration Determination with MicroED
MicroED has also been integrated with chiral salts to help resolve the absolute configuration of small molecules. This is an important advancement in the field of structural chemistry, where determining the configuration of molecules is often a significant challenge.
Cross-disciplinary Integration in MicroED
Researchers are increasingly combining MicroED with molecular dynamics simulations and machine learning techniques to study protein dynamics and molecular interactions in greater detail, opening up new possibilities for drug design and biomolecular research.
What's Next for MicroED Technology?
- MicroED for Dynamic Structural Studies: The future of MicroED lies in its ability to capture dynamic structural changes through time-resolved techniques. This will allow researchers to observe conformational shifts in proteins and other biomolecules in response to various stimuli, such as binding events or environmental changes.
- In Situ Structural Analysis with MicroED: Another promising direction for MicroED is the ability to analyze protein complexes and other biomolecules directly within their native environments, such as within cells or lipid membranes. This in situ approach will provide more accurate structural data that reflects the biological context of the samples.
- High-Throughput Drug Screening Using MicroED: MicroED's high-resolution capabilities make it an ideal tool for high-throughput drug screening, especially for targets that are difficult to crystallize. The ability to analyze small crystals quickly and efficiently could accelerate structure-based drug design and lead to the discovery of new therapeutics.
MicroED is proving to be an indispensable tool for both structural biology and materials science, enabling the detailed analysis of small molecules, biomolecules, and complex materials. With its ability to resolve structures from micro-sized crystals and even powders, it opens up new possibilities for research in drug discovery, materials engineering, and beyond. If you're interested in learning more about how MicroED can enhance your research or are looking for professional services, visit our microcrystal electron diffraction (MicroED) services at Creative Biostructure. Our experts are ready to help you harness the power of MicroED to drive your scientific advancements forward.
References
- Hattne J, Reyes F E, Nannenga B L, et al. MicroED data collection and processing. Foundations of Crystallography. 2015, 71(4): 353-360.
- Jones C G, Martynowycz M W, Hattne J, et al. The CryoEM method MicroED as a powerful tool for small molecule structure determination. ACS Central Science. 2018, 4(11): 1587-1592.
- Martynowycz M W, Zhao W, Hattne J, et al. Collection of continuous rotation MicroED data from ion beam-milled crystals of any size. Structure. 2019, 27(3): 545-548. e2.
- Xu H, Lebrette H, Clabbers M T B, et al. Solving a new R2lox protein structure by microcrystal electron diffraction. Science Advances. 2019, 5(8): eaax4621.
- Nannenga B L, Gonen T. The cryo-EM method microcrystal electron diffraction (MicroED). Nature Methods. 2019, 16(5): 369-379.
- Nguyen C, Gonen T. Beyond protein structure determination with MicroED. Current Opinion in Structural Biology. 2020, 64: 51-58.
- Martynowycz M W, Shiriaeva A, Ge X, et al. MicroED structure of the human adenosine receptor determined from a single nanocrystal in LCP. Proceedings of the National Academy of Sciences. 2021, 118(36): e2106041118.
- Mu X, Gillman C, Nguyen C, et al. An overview of microcrystal electron diffraction (MicroED). Annual Review of Biochemistry. 2021, 90(1): 431-450.
- Meng Z, Jones C G, Farid S, et al. Unraveling the electrical and magnetic properties of layered conductive metal‐organic framework with atomic precision. Angewandte Chemie International Edition. 2022, 61(6): e202113569.
- Haymaker A, Bardin A A, Gonen T, et al. Structure determination of a DNA crystal by MicroED. Structure. 2023, 31(12): 1499-1503. e2.

-1.jpg)