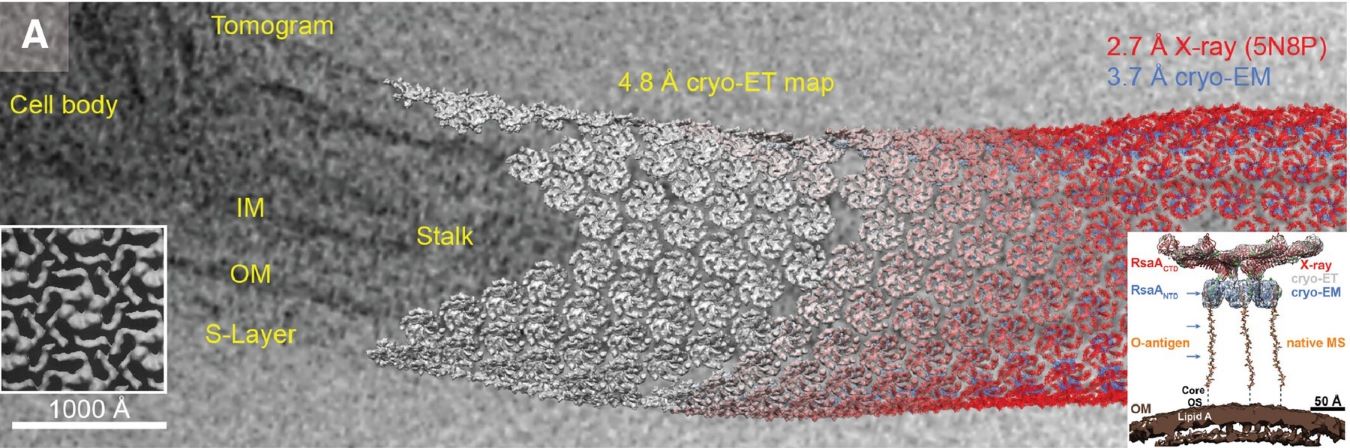
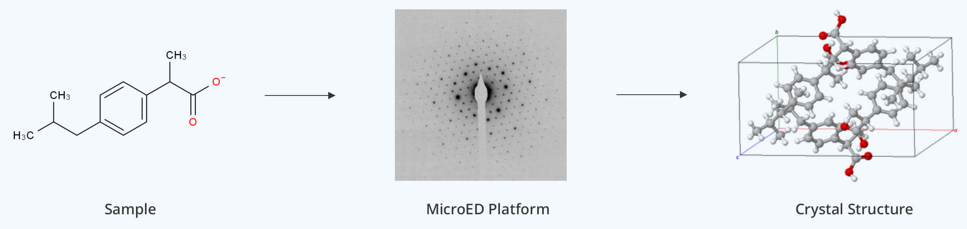
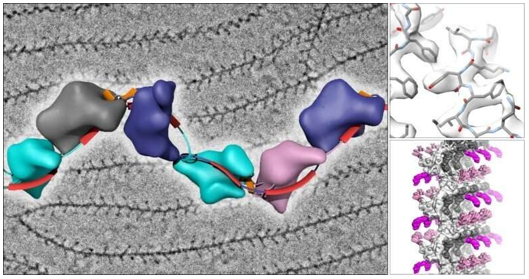
In structural biology, high-resolution 3D models of macromolecules are essential for understanding biological functions and developing new therapies. X-ray crystallography has long been the standard method, but it requires large, high-quality crystals—often difficult to obtain, especially for membrane proteins and flexible molecules.
To address these challenges, Single Particle Analysis (SPA) and Microcrystal Electron Diffraction (MicroED) have emerged as powerful cryo-EM techniques. SPA earned the 2017 Nobel Prize in Chemistry, while MicroED was named a top scientific breakthrough in 2018. But how do they compare? This article explores their differences, resolution capabilities, advantages, and which is best suited for your research.
What is MicroED Technology?
MicroED is an emerging technique that combines cryo-electron microscopy with crystallography to study the atomic structure of biomolecules from sub-micron-sized crystals. It uses a transmission electron microscope to collect 3D diffraction data from tiny crystals, typically on the scale of nanometers to micrometers. By analyzing the electron diffraction patterns, researchers can determine the atomic-level structure of the biomolecules using X-ray crystallography software.
What is Cryo-EM SPA Technology?
Cryo-EM SPA is a technique that allows researchers to study the three-dimensional structures of biomolecules by imaging frozen, non-crystalline particles. In this approach, individual particles are rapidly frozen in a vitreous ice state, and thousands to millions of 2D projection images are captured. These images are then aligned, classified, and used to reconstruct the 3D structure of the biomolecule at near-atomic resolution.
Select Service
MicroED Application Scenarios and Advantages
MicroED is a cutting-edge technique that enables the determination of atomic-level structures from very small crystals, a challenge for traditional X-ray crystallography. It has proven to be particularly effective in the following areas:
Protein Structure Research
MicroED has shown great potential in protein structure research, particularly for proteins that are difficult to crystallize. By determining high-resolution structures from nanoscale crystals, MicroED enables the analysis of proteins that are otherwise challenging to study using conventional X-ray techniques.
For instance, MicroED has successfully resolved the structures of the human adenosine receptor and core structures of α-synuclein, providing insights into protein folding, function, and interactions. This is particularly important for understanding proteins involved in neurodegenerative diseases like Parkinson's disease, where traditional crystallography methods often fall short.
Small Molecule Structure Determination
MicroED also excels in small molecule structure determination. By collecting high-resolution diffraction data from very small crystals, it is capable of providing detailed atomic-level insights into pharmaceutical compounds. This is particularly beneficial for drug development and designing new therapeutic agents.
Examples of MicroED applications include the structural determination of pharmaceutical compounds like progesterone, ibuprofen, and acetaminophen, which are key to drug development. The ability to obtain high-resolution data from microcrystals accelerates the analysis and optimization of small-molecule drugs.
Natural Product Research
MicroED is particularly useful for studying natural products, which often involve complex molecular structures that are difficult to analyze with traditional methods. This technique allows researchers to extract detailed structural information from tiny crystals, even in challenging samples.
For example, MicroED has been applied in studying biomineralization processes in plants, uncovering the atomic structures of minerals like calcium-sodium chloride crystals formed during plant metabolism. This opens up new pathways for exploring bioactive natural compounds with potential pharmaceutical applications.
Drug Development
MicroED's ability to quickly determine the structure of small molecules makes it a powerful tool in drug development. By providing high-resolution structures of drug candidates and their interactions with target proteins, MicroED helps to accelerate the drug design process.
For example, the structure of catalytic enzymes has been successfully determined using MicroED, facilitating the development of enzyme inhibitors. The technique shortens the timeline for drug development, enabling faster identification of promising drug candidates and improving the efficacy of therapeutic compounds.
MicroED Case Studies
In the early stages of MicroED development, several model samples, such as lysozyme, catalase, and Ca²⁺-ATPase, were successfully solved at atomic resolution. As the technique matured, it was no longer limited to model samples, and researchers began to explore the structures of unknown proteins, such as the α-synuclein core peptide and R2lox enzyme, achieving resolutions as high as ~1 Å.
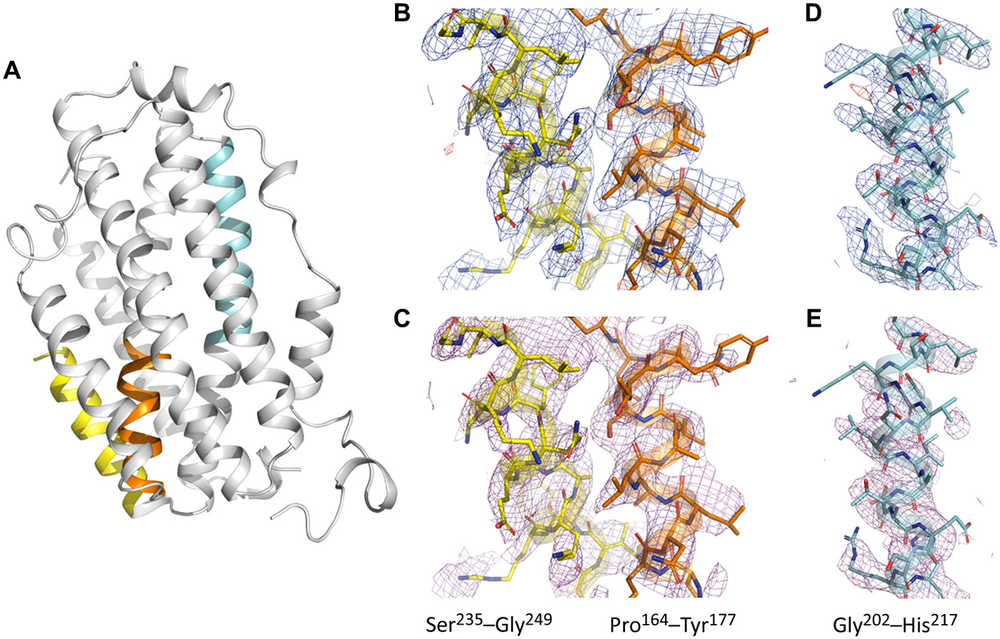 Figure 1. (A) Overall structure of SaR2lox solved by MicroED, with color-coded selections showing electrostatic potential maps in (B)-(E). (B)-(E) Electrostatic maps include 2Fo − Fc (blue, 1σ), Fo − Fc (green/red for positive/negative peaks at ±3σ), and simulated annealing composite omit maps (magenta, 1σ). The maps for residues 164-177 (orange), 235-249 (yellow), and 202-217 (cyan) demonstrate high-quality, well-resolved data despite low completeness. (Xu H, et al., 2019)
Figure 1. (A) Overall structure of SaR2lox solved by MicroED, with color-coded selections showing electrostatic potential maps in (B)-(E). (B)-(E) Electrostatic maps include 2Fo − Fc (blue, 1σ), Fo − Fc (green/red for positive/negative peaks at ±3σ), and simulated annealing composite omit maps (magenta, 1σ). The maps for residues 164-177 (orange), 235-249 (yellow), and 202-217 (cyan) demonstrate high-quality, well-resolved data despite low completeness. (Xu H, et al., 2019)
Following 2016, MicroED experienced rapid advancements, with a significant increase in the number of protein structures solved using this technique. High-resolution structures around 1 Å were obtained for samples like prions and FUS LC (fused in sarcoma low-complexity domain). As the technology progressed, more challenging targets were tackled, including protein complexes and membrane proteins, such as ion channels.
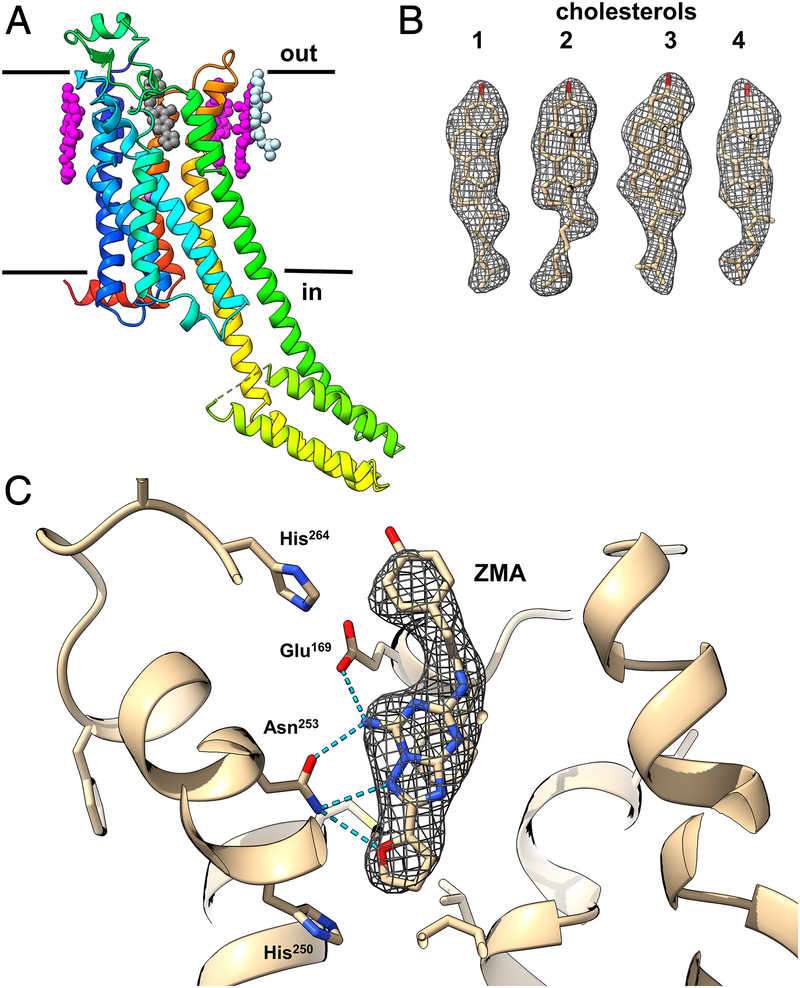 Figure 2. Structure of A2AAR GPCR Revealed by MicroED. (A) Cartoon representation of A2AAR structure in rainbow colors, showing the membrane bilayer and ligand positions (ZM241385 in black, cholesterol in magenta and light blue). (B) Density map for the four cholesterol molecules. (C) Magnified view of the ZM241385 antagonist binding pocket, showing protein-ligand coordination. (Martynowycz M W, et al., 2021)
Figure 2. Structure of A2AAR GPCR Revealed by MicroED. (A) Cartoon representation of A2AAR structure in rainbow colors, showing the membrane bilayer and ligand positions (ZM241385 in black, cholesterol in magenta and light blue). (B) Density map for the four cholesterol molecules. (C) Magnified view of the ZM241385 antagonist binding pocket, showing protein-ligand coordination. (Martynowycz M W, et al., 2021)
Beyond biological macromolecules, MicroED has proven particularly well-suited for studying chemical small molecules, natural products, and pharmaceutical formulations. Small molecules often present as powders, making them ideal candidates for MicroED, which can efficiently analyze their structures. Numerous successful cases of chemical small molecule research have validated the technique's high efficiency and broad applicability, further cementing MicroED's potential in drug discovery and chemical analysis.
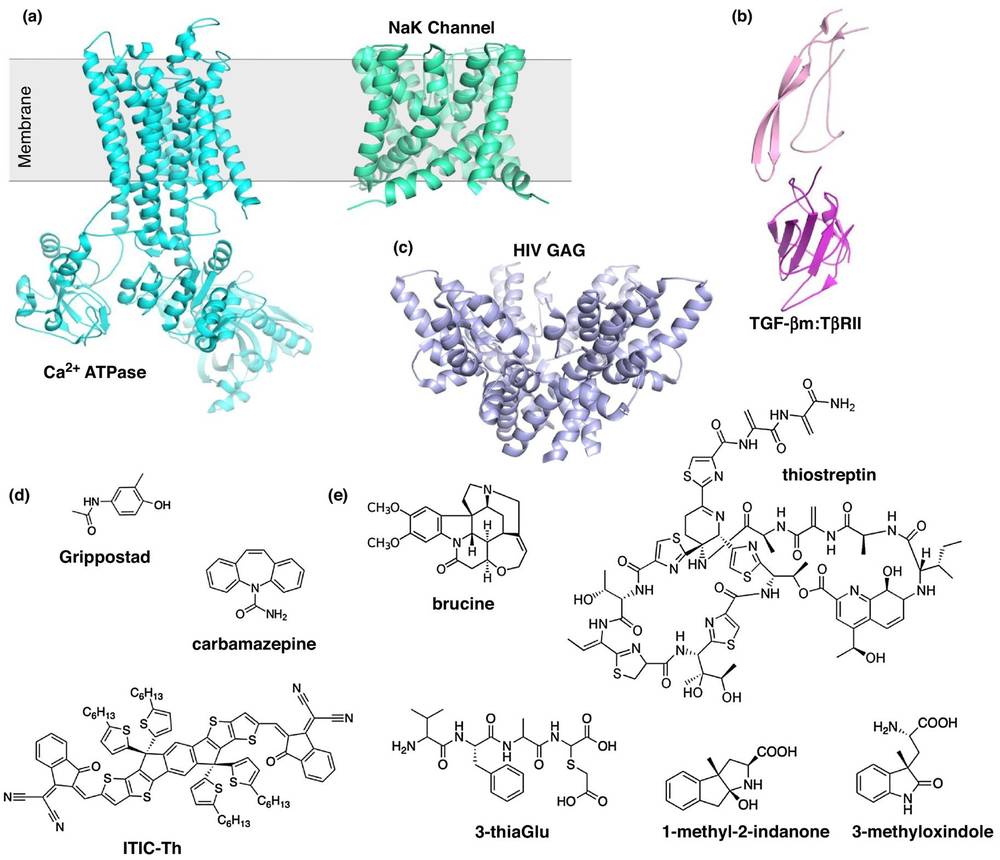 Figure 3. MicroED Structures of Membrane Proteins, Complexes, Small Molecules, and Natural Products. (a) MicroED structures of Ca2+ ATPase (cyan) and NaK (green) membrane proteins. (b) MicroED structure of the TGF-βm:TβRII protein–protein complex. (c) MicroED structure of HIV-GAG-bevirimat complex. (d) Gallery of small molecules determined by MicroED. (e) Natural products with structures solved using MicroED. (Nguyen C, et al., 2020)
Figure 3. MicroED Structures of Membrane Proteins, Complexes, Small Molecules, and Natural Products. (a) MicroED structures of Ca2+ ATPase (cyan) and NaK (green) membrane proteins. (b) MicroED structure of the TGF-βm:TβRII protein–protein complex. (c) MicroED structure of HIV-GAG-bevirimat complex. (d) Gallery of small molecules determined by MicroED. (e) Natural products with structures solved using MicroED. (Nguyen C, et al., 2020)
Key Advantages of MicroED
MicroED offers several key advantages over traditional methods, particularly for small and difficult-to-crystallize samples:
- High-Resolution Structure Determination: MicroED provides high-resolution data from very small crystals, overcoming the need for large crystals required in X-ray crystallography. Its ability to resolve finer details is due to the shorter wavelength of electrons, making it ideal for studying proteins, small molecules, and delicate biological samples.
- Small Crystal Applicability: MicroED can analyze crystals as small as a few hundred nanometers, making it highly effective for small, complex molecules and biological samples that cannot be easily crystallized by conventional methods.
- Wide Applicability: MicroED can be used for a variety of samples, including proteins, small molecules, DNA, and biominerals, making it versatile for both basic and applied research, including drug development and material science.
- Increased Success in Co-crystal Formation: MicroED shows a higher success rate for forming co-crystals of proteins and small molecules, critical for drug development. Smaller crystal sizes make it easier to incorporate small molecules, improving co-crystal formation without damaging the crystal.
- No Need for X-ray Molecular Replacement (MR) Data: Unlike X-ray crystallography, MicroED solves structures directly from electron diffraction data, eliminating the need for molecular replacement techniques. This simplifies the process and extends its applicability to challenging samples.
- Lower Equipment Requirements: MicroED can be performed with relatively modest equipment, such as lower-end transmission electron microscopes (TEMs), making it more accessible and cost-effective. Data collection is quick, often taking only minutes, even for complex datasets.
- Automation and High-Throughput Capability: MicroED's data collection can be automated, enabling high-throughput analysis for large-scale studies or rapid sample screening. This speeds up the process and ensures consistent, reproducible results, which is especially useful in drug discovery.
Cryo-EM SPA Application Scenarios and Advantages
Protein Structure Determination
Cryo-EM SPA is widely used for high-resolution structure determination of proteins and their complexes. It provides atomic-level details of three-dimensional structures, allowing researchers to understand protein functions and interactions at the molecular level.
Pathogen Research
The technology is particularly useful in pathogen biology, especially in viral structure analysis. For example, in 2020, Cryo-EM SPA was used to determine the stable trimeric conformation of the SARS-CoV-2 spike protein, providing crucial information for vaccine development.
Drug Discovery
By solving the high-resolution structures of drug targets, Cryo-EM SPA offers new insights into drug design and development. It helps researchers understand how small molecules bind to targets, facilitating the optimization of drug efficacy and specificity.
Cell Biology
Cryo-EM SPA is increasingly applied in cell biology to study large molecular complexes inside cells, such as ribosomes and viral particles. This provides essential information about cellular mechanisms and molecular functions in a near-native state.
Cryo-EM SPA Case Studies
Single Particle Cryo-EM has revolutionized structural biology by enabling the study of proteins and macromolecular complexes without the need for crystallization, which is particularly valuable for challenging targets like membrane proteins and large molecular complexes. This method requires only small quantities of samples, making it ideal for studying difficult-to-crystallize proteins and complex biological systems, such as membrane proteins, antibody-epitope mapping, and AAV capsids.
A notable case using Cryo-EM SPA is the determination of human glucagon receptor (GCGR) structures at 3.7 Å and 3.9 Å resolution, which were bound to glucagon and different types of heterotrimeric G-proteins (Gs or Gi1). These findings provided critical insights into receptor activation mechanisms and were instrumental in identifying potential therapeutic strategies for Type II diabetes and metabolic disorders. The integration of multiple structural and pharmacological data opened new avenues for drug design and treatment development.
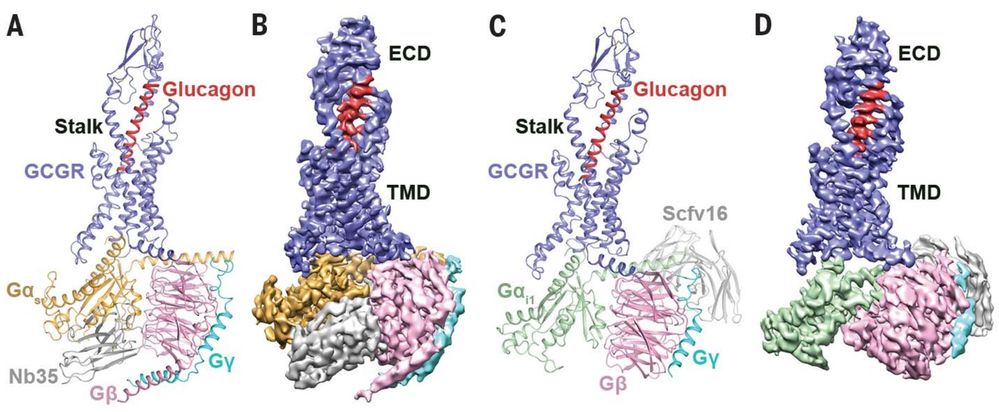 Figure 4. Cryo-EM Reveals Structures of Glucagon-GCGR-G Protein Complexes. (A) Cryo-EM structure of glucagon-GCGR-Gₛ-Nb35 complex, with Nb35 stabilizing the Gαₛ-Gβ interface. (B) Cryo-EM map of the glucagon-GCGR-Gₛ-Nb35 complex. (C) Cryo-EM structure of glucagon-GCGR-Gᵢ₁-Scfv16 complex, with Scfv16 stabilizing the GPCR-Gᵢ complex. (D) Cryo-EM map of glucagon-GCGR-Gᵢ₁-Scfv16 complex. (Qiao A, et al., 2020)
Figure 4. Cryo-EM Reveals Structures of Glucagon-GCGR-G Protein Complexes. (A) Cryo-EM structure of glucagon-GCGR-Gₛ-Nb35 complex, with Nb35 stabilizing the Gαₛ-Gβ interface. (B) Cryo-EM map of the glucagon-GCGR-Gₛ-Nb35 complex. (C) Cryo-EM structure of glucagon-GCGR-Gᵢ₁-Scfv16 complex, with Scfv16 stabilizing the GPCR-Gᵢ complex. (D) Cryo-EM map of glucagon-GCGR-Gᵢ₁-Scfv16 complex. (Qiao A, et al., 2020)
Another significant breakthrough involves the study of antibody recognition epitopes. Using Cryo-EM SPA, the three-dimensional structure of the human parechovirus 3 (HPeV3) surface epitope cluster and its complex with antibodies was solved (EMD-0069). This structural data is essential for understanding antibody binding and will help guide the development of targeted antiviral therapies. These examples highlight how Cryo-EM SPA can solve intricate biological structures that are otherwise challenging to crystallize, paving the way for advancements in drug discovery, vaccine design, and molecular biology.
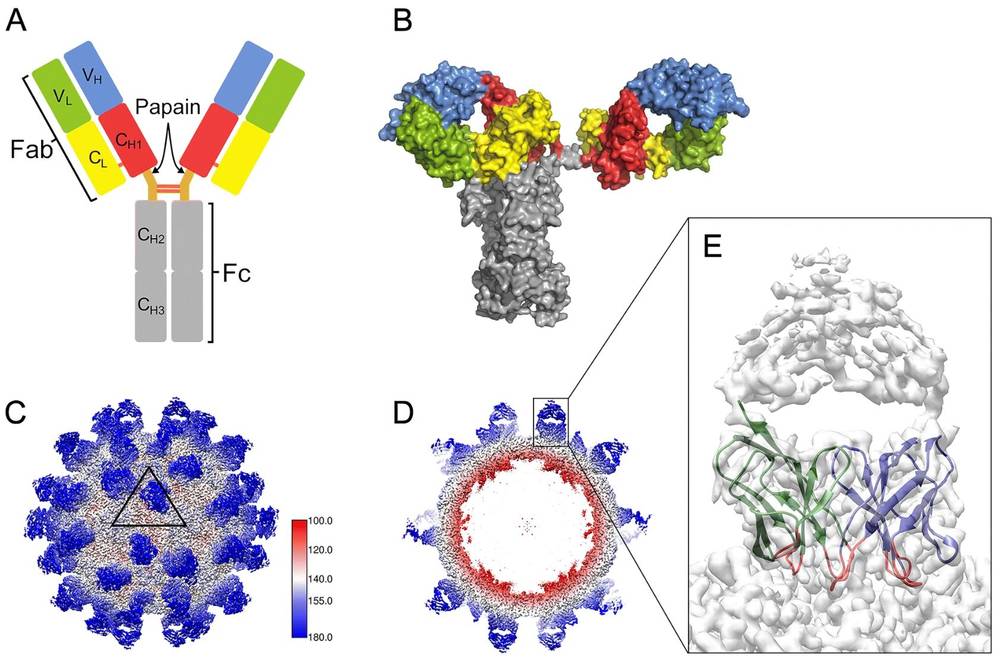 Figure 5. Cryo-EM Analysis of Antibody Molecules on Viral Surfaces. (A) Modular organization of a Y-shaped IgG molecule with heavy and light chains. (B) Surface illustration of human IgG1 b12. (C) Cryo-EM map of Fab AT12-015 complexed with HPeV3, showing 60 Fab copies on the viral surface. (D) Central cross-section of the cryo-EM map showing Fab densities. (E) Magnified view showing Fab AT12-015 with well-defined VH and VL domains. (Li N, et al., 2020)
Figure 5. Cryo-EM Analysis of Antibody Molecules on Viral Surfaces. (A) Modular organization of a Y-shaped IgG molecule with heavy and light chains. (B) Surface illustration of human IgG1 b12. (C) Cryo-EM map of Fab AT12-015 complexed with HPeV3, showing 60 Fab copies on the viral surface. (D) Central cross-section of the cryo-EM map showing Fab densities. (E) Magnified view showing Fab AT12-015 with well-defined VH and VL domains. (Li N, et al., 2020)
Key Advantages of Cryo-EM SPA
- High-Resolution Imaging: Cryo-EM SPA can achieve near-atomic resolution, which is crucial for understanding the intricate structures of biological molecules, offering insights that traditional methods may not reveal.
- In Situ Imaging: This technique allows for imaging in a near-physiological environment, preserving the native state of biological molecules without introducing structural changes that may occur during traditional fixation or staining methods.
- Multi-Scale Imaging: By combining sub-micron resolution cryo-ET and single-particle analysis, Cryo-EM SPA can achieve multi-scale imaging, providing a comprehensive view of complex biological systems across different size scales.
- Non-Destructive: Cryo-EM SPA is non-destructive, enabling repeated imaging of the same sample without altering it. This is particularly useful for rare or difficult-to-obtain samples, as well as for long-term studies of dynamic processes.
- Flexibility: With advances in hardware and algorithms, Cryo-EM SPA has become increasingly flexible, applicable to a wide range of biological samples, including membrane proteins and large molecular complexes. This adaptability makes it a valuable tool for diverse areas of biological research.
Select Service
MicroED vs. Cryo-EM SPA: Resolution Differences
1. MicroED Resolution Range
- Typical Range: 1.0-3.2 Å, with some cases reaching sub-Å precision (e.g., 0.73 Å for FUS protein amyloid core).
- High-Resolution Breakthroughs: Through Continuous Rotation MicroED (CR-MicroED) and high-speed detectors, MicroED can resolve atomic-level details of small molecules, peptides, and proteins (e.g., 1.4 Å structure of α-synuclein).
- Dynamic Scattering Limitations: Crystal thickness and dynamic scattering effects may limit resolution, but optimizing crystal thickness through focused ion beam (FIB) milling (e.g., 200 nm) can enhance resolution to 2.8 Å (e.g., A2A adenosine receptor structure).
2. Cryo-EM SPA Resolution Range
- Mainstream Range: 2-4 Å, with some smaller proteins (e.g., ferritin) reaching up to 1.9 Å.
- Technological Advancements: Direct electron detectors (DED) and Bayesian motion correction algorithms have enabled near-atomic resolution for large complexes, such as the 3.5 Å structure of the 2019-nCoV spike protein.
- Challenges for Small Molecules: Resolution for molecules under 100 kDa is still limited (e.g., sub-nanometer structure of the 43 kDa protein kinase A).
Resolution Summary Comparison Table
| Technology | Best Resolution | Applicable Molecular Size | Dynamic Range Advantage |
|---|---|---|---|
| MicroED | 0.73–3.2 Å | Small molecules to large proteins | High precision for small (micron-sized) crystals |
| Cryo-EM SPA | 1.2–4.0 Å | Primarily >100 kDa | No crystallization required, ideal for large complexes |
MicroED vs. Cryo-EM SPA: Sample Preparation Requirements
1. MicroED Sample Preparation
- Crystal Requirements: MicroED requires micro-sized three-dimensional crystals (approximately 0.5-1 μm), which can be obtained using traditional crystallization conditions or lipidic cubic phase (LCP) crystallization methods.
- Key Steps
1) Crushing and Screening: Large crystals are fragmented via sonication or pipetting, followed by screening for suitable microcrystals.
2) FIB Milling: For membrane protein crystals (e.g., GPCRs), LCP is converted to a sponge phase, and crystals are milled to 200 nm thick slices using focused ion beam (FIB).
3) Cryo-Protectants: Cryoprotectants like PEG400 are used to prevent dehydration damage during freezing. - Equipment Requirements: TEM, carbon-coated grids, and liquid ethane freezing equipment are necessary.
2. Cryo-EM SPA Sample Preparation
- Amorphous Requirements: In contrast, cryo-EM SPA requires highly purified, homogeneous samples. These particles should be in a single dispersion state and prepared for vitrification. Cryo-EM is sensitive to the concentration and size distribution of particles, and a high level of sample homogeneity is necessary to produce clear and high-resolution reconstructions. Purified particles need to be evenly distributed on a thin ice layer, avoiding aggregation or adsorption to the air-water interface.
- Process Optimization
1) Blot-less Method: Eliminates mechanical stress, improving the distribution of particles.
2) Jet-based Vitrification: Fast freezing to preserve the natural conformation of the sample.
3) Pre-screening: Low-temperature light microscopy is used to identify high-quality ice areas. - Challenges: Low-concentration samples may result in thick ice layers, requiring optimization of buffer composition (e.g., adding glycerol).
Learn more in single particle cryo-EM sample preparation guide.
Sample Preparation Summary Comparison Table
| Technology | Sample Form | Equipment Complexity | Special Treatment Needs |
|---|---|---|---|
| MicroED | Microcrystals (ordered) | High (requires FIB) | Crystal fragmentation, lipid phase conversion |
| Cryo-EM SPA | Single particles (disordered) | Medium | Ice layer uniformity, particle dispersion |
MicroED vs. Cryo-EM SPA: Data Processing Methods
1. MicroED Data Processing
- Software Tools: MicroED uses X-ray crystallography software such as XDS, PHASER, and PHENIX for integration, merging, and refinement of the data.
- Key Processes
1) Continuous Rotation Data Integration: This process helps reduce the effects of dynamic scattering and improves the completeness of reflections.
2) Dynamic Scattering Correction: Crystal thickness (e.g., 185 nm) is optimized through simulations to correct for dynamic scattering effects.
3) Phase Determination: Molecular replacement or homologous models are used for phase determination. In the future, direct electron scattering factors may be integrated for phase determination.
2. Cryo-EM SPA Data Processing
- Software Tools: Cryo-EM SPA data is typically processed using software like RELION and CryoSPARC, with support for GPU acceleration.
- Key Processes
1) Motion Correction and CTF Estimation: Bayesian methods are used to optimize particle trajectories and correct for aberrations.
2) 3D Classification and Refinement: Local symmetry constraints and Ewald sphere corrections improve the resolution of large macromolecular complexes.
3) Local Resolution Analysis: Identifies flexible regions within structures (e.g., ribosomal RNA).
Learn more in data processing in cryo-EM single particle analysis.
Data Processing Summary Comparison Table
| Technology | Main Software | Computational Complexity | Algorithm Innovations |
|---|---|---|---|
| MicroED | XDS/PHENIX | Low (similar to X-ray) | Dynamic scattering modeling, phase methods |
| Cryo-EM SPA | RELION/CryoSPARC | High (requires GPU) | Motion correction, heterogeneity analysis |
MicroED vs. Cryo-EM SPA: Advantages and Limitations
1. MicroED Advantages
- Small Crystal Applicability: MicroED can solve structures from microcrystals that traditional X-ray crystallography cannot handle, such as lipid-bilayer membrane proteins.
- Atomic-Level Charge Analysis: Electron scattering factors offer more accurate charge density information, crucial for identifying inhibitor binding sites.
- High-Throughput Potential: Single-crystal data collection is fast (approximately 1 hour), making it suitable for drug screening.
2. MicroED Limitations
- Crystal Quality Dependency: Dynamic scattering and lattice defects can limit the resolution of the structure.
- Equipment Requirements: Requires high-end TEM and FIB milling systems for sample preparation and data collection.
3. Cryo-EM SPA Advantages
- No Crystallization Required: Cryo-EM SPA can analyze flexible complexes, such as viral capsids and ribosomes, without the need for crystallization.
- Conformational Heterogeneity Analysis: 3D classification allows for the capture of dynamic conformational changes, providing detailed insight into molecular mechanisms.
4. Cryo-EM SPA Limitations
- Signal-to-Noise Ratio Limitation: Resolution decreases significantly for small molecules (<100 kDa), which limits its use for small molecules.
- Data Quantity Requirement: Requires thousands to millions of particle images for accurate reconstruction.
Summary
SPA and MicroED each play a vital role in structural biology. Cryo-EM SPA is ideal for large, flexible complexes and membrane proteins, bypassing the need for crystallization. It typically achieves ~3 Å resolution, though advances have pushed it below 2 Å for some cases. However, it struggles with small molecules and requires extensive data collection.
MicroED, on the other hand, excels in high-resolution analysis, reaching atomic-level detail (1 Å for small molecules, 2 Å for larger ones) from tiny crystals unsuitable for X-ray diffraction. With cryo-FIB, it can process larger crystals and offers faster data collection with fewer resources, making it especially useful in drug discovery.
Comparison Summary Table of SPA and MicroED
| Aspect | Single Particle Analysis | MicroED |
|---|---|---|
| Typical Sample Types | Membrane proteins, antibodies, viruses | Membrane proteins, protein-drug complex, small molecules |
| Protein Size Requirement | >150KDa | None |
| Crystal Size Requirement | No crystallization needed | 100 nm crystals are sufficient |
| Sample Amount Requirement | Very small amount (about 0.1mg) | Small amount (several mg level) |
| Resolution | Large molecule samples, ~3 Å (lower resolution, not conducive to resolving small molecule binding modes) | Large molecule samples, ~2 Å (can resolve small molecule binding modes) |
| Testing Time | Data collection time of several days | Data collection time of 1-2 hours |
Technical Selection Recommendations
- Prioritize MicroED: When the sample easily forms microcrystals (e.g., membrane proteins or small molecule complexes) and requires atomic-level precision.
- Prioritize Cryo-EM SPA: When the sample is difficult to crystallize or requires analysis of conformational heterogeneity (e.g., flexible complexes or viral particles).
In conclusion, MicroED excels in high-resolution studies of small molecules and challenging-to-crystallize proteins, while Cryo-EM SPA is ideal for large, flexible macromolecular complexes. The choice depends on research goals, sample type, and resolution needs. Used together, they offer a comprehensive view—from atomic details to large-scale conformational changes. For instance, MicroED can reveal binding sites, while Cryo-EM SPA captures overall structural dynamics.
If you're looking to explore the potential of microcrystal electron diffraction or single particle cryo-EM service for your research, Creative Biostructure offers expert solutions to accelerate your discoveries. Contact us for expert consultation and access to cutting-edge structural analysis technologies.
References
- Liu S, Hattne J, Reyes F E, et al. Atomic resolution structure determination by the cryo‐EM method MicroED. Protein Science. 2017, 26(1): 8-15.
- Fan X, Wang J, Zhang X, et al. Single particle cryo-EM reconstruction of 52 kDa streptavidin at 3.2 Angstrom resolution. Nature Communications. 2019, 10(1): 2386.
- Martynowycz M W, Zhao W, Hattne J, et al. Collection of continuous rotation MicroED data from ion beam-milled crystals of any size. Structure. 2019, 27(3): 545-548. e2.
- Xu H, Lebrette H, Clabbers M T B, et al. Solving a new R2lox protein structure by microcrystal electron diffraction. Science Advances. 2019, 5(8): eaax4621.
- Yip K M, Fischer N, Paknia E, et al. Atomic-resolution protein structure determination by cryo-EM. Nature. 2020, 587(7832): 157-161.
- Nguyen C, Gonen T. Beyond protein structure determination with MicroED. Current Opinion in Structural Biology. 2020, 64: 51-58.
- Li N, Li Z, Fu Y, et al. Cryo-EM studies of virus-antibody immune complexes. Virologica Sinica. 2020, 35(1): 1-13.
- Qiao A, Han S, Li X, et al. Structural basis of Gs and Gi recognition by the human glucagon receptor. Science. 2020, 367(6484): 1346-1352.
- Martynowycz M W, Shiriaeva A, Ge X, et al. MicroED structure of the human adenosine receptor determined from a single nanocrystal in LCP. Proceedings of the National Academy of Sciences. 2021, 118(36): e2106041118.

-1.jpg)