What Is Single Particle Cryo-EM Sample Preparation
Single particle cryo-electron microscopy (cryo-EM) is a powerful technique that can be used to determine the three-dimensional structure of biological macromolecules at near-atomic resolution. Sample preparation is a critical step in this workflow as it directly affects the quality of the final structure determined by cryo-EM.
Importance of Sample Preparation in Single Particle Cryo-EM
Sample preparation is one of the key steps for the success of single particle cryo-EM technology. The importance of sample preparation is reflected in the following aspects:
- Sample purity and homogeneity: In order to obtain a high-resolution structure, the sample must have a high degree of purity and homogeneity. This usually requires a biochemical purification step to remove impurities and non-target proteins and ensure sample stability in solution.
- Sample stability: Samples must remain in their natural state before freezing to avoid structural changes due to environmental changes. For example, proteins may denature or aggregate at the air-water interface (AWI), which may affect subsequent structural resolution.
- Uniform distribution of samples: During the preparation process, the samples need to be evenly distributed on the grid of the electron microscope and form a thin layer of vitreous ice. This step is critical to reducing scattering events during imaging.
- Diversified application needs: Different types of biological samples (such as membrane proteins, viruses, nucleic acid complexes, etc.) have different requirements for sample preparation. Therefore, researchers need to select appropriate preparation methods and tools based on specific research goals.
Overview of Single Particle Cryo EM Sample Preparation Workflow
The workflow of single particle cryo-EM sample preparation involves several key steps, each of which is critical to achieving high-quality imaging and accurate structural analysis. The following is a detailed breakdown of a typical workflow:
- Protein Expression
- Protein Purification Optimization
- Cryo-EM Sample Quality Assessment
- Cryo-EM Vitrification
- Cryo-EM Grid Screening and Optimization
Each step directly influences the quality of the final structure determination. The process begins with selecting an appropriate expression system, continues through biochemical methods to ensure sample purity and homogeneity, proceeds to rapid freezing to form vitreous ice, and concludes with preliminary microscopic evaluation - these steps are interconnected and indispensable. Now, let's delve into the specific details and considerations for each crucial step in this workflow.
Protein Expression and Purification: The Foundation of High-Quality Cryo-EM Samples
The success of cryo-EM structural studies heavily depends on the quality of the protein sample. Unlike crystallography, where crystal packing can sometimes stabilize certain protein conformations, cryo-EM requires exceptionally pure, homogeneous, and stable protein samples in their native state. This fundamental requirement makes protein expression and purification particularly crucial for cryo-EM applications.
Expression Systems Selection
The choice of expression system significantly impacts the final sample quality for cryo-EM analysis:
| Expression Systems | Features |
|---|---|
| Prokaryotic Expression Systems |
|
| Eukaryotic Expression Systems |
|
| Cell-free Expression Systems |
|
Select Service
Purification Strategies for Cryo-EM Samples
To achieve high-resolution results in cryo-EM studies, particularly with single particle analysis, it is crucial to address sample heterogeneity through 3D classification techniques. However, this alone is insufficient; biochemical purification of the target protein is essential before imaging. This purification step involves eliminating impurities and contaminants that could compromise image quality. Methods such as affinity chromatography are commonly employed to isolate the target protein from other sample components. The purification process for cryo-EM samples demands specific considerations:
Multi-step Purification Approach
A systematic, multi-step purification strategy is essential for achieving the high sample quality required for cryo-EM analysis, typically involving a sequence of complementary chromatographic techniques.
1. Initial Capture: Affinity Chromatography
The first step leverages specific molecular interactions between tagged proteins and immobilized ligands, offering highly selective isolation of the target protein from crude cell lysates with typical yields of 90% or higher purity in a single step.
- Tag Selection and Optimization
Strategic selection of affinity tags (such as His-tag, GST, MBP, or FLAG) is crucial for maximizing protein solubility and enabling efficient purification while maintaining native protein structure and function.
- Tag Removal Considerations
Implementation of specific protease cleavage sites enables tag removal under controlled conditions, reducing potential interference with protein structure and function during cryo-EM analysis.
2. Intermediate Purification: Ion Exchange Chromatography
This step separates proteins based on their surface charge characteristics, effectively removing contaminants that co-purified during affinity chromatography and further increasing sample purity to >95%.
- Buffer Optimization for Ion Exchange
Careful optimization of pH and salt concentration gradients ensures optimal separation while maintaining protein stability and preventing aggregation during the purification process.
- Polishing: Size Exclusion Chromatography
The final polishing step separates proteins based on their hydrodynamic radius, effectively removing any remaining aggregates, degradation products, and buffer components while simultaneously assessing sample homogeneity.
- Critical SEC Parameters
Selection of appropriate column matrix, flow rate, and buffer conditions is essential for achieving optimal separation while maintaining protein stability and integrity.
Quality Control Checkpoints
Implementation of rigorous quality control measures at each purification step, including SDS-PAGE, Western blot, and activity assays, ensures consistent sample quality and helps identify potential issues early in the purification process.
- Sample Stability Assessment
Regular monitoring of protein stability through techniques such as dynamic light scattering and thermal stability assays helps optimize storage conditions and predict sample behavior during cryo-EM grid preparation.
- Documentation and Reproducibility
Maintaining detailed records of purification parameters, yields, and quality metrics at each step ensures reproducibility and facilitates troubleshooting when needed.
- Final Quality Standards
Establishment of clear acceptance criteria for final sample quality, including purity thresholds, stability requirements, and functional activity benchmarks, ensures only high-quality samples proceed to cryo-EM analysis.
Critical Parameters for Optimization
- Buffer Composition and Stability
The careful selection of buffer components, including pH range (typically 6.5-8.0), salt concentration (usually 150-300 mM), and specific additives (such as glycerol, detergents, or stabilizing agents), plays a crucial role in maintaining protein stability and preventing aggregation during purification and grid preparation.
- Temperature Control Strategies
Maintaining consistent and appropriate temperature conditions throughout the purification process is essential for preventing protein degradation and maintaining sample integrity, with specific attention to temperature-sensitive steps and storage conditions.
- Aggregation and Degradation Prevention
Implementation of preventive measures against protein aggregation and degradation, such as optimizing protein concentration, adding stabilizing agents, and minimizing freeze-thaw cycles, ensures sample quality and homogeneity for successful cryo-EM analysis.
- Concentration Optimization for Grid Preparation
The final protein concentration must be carefully optimized (typically ranging from 0.1-5 mg/mL) to achieve ideal particle distribution on cryo-EM grids while avoiding concentration-dependent aggregation or other adverse effects that could compromise structural analysis.
Select Service
Related Reading
- What Is Chromatography?
- High-Performance Liquid Chromatography (HPLC)
- Reversed-Phase Chromatography (RPC)
- Hydrophobic Interaction Chromatography (HIC)
- What Do Chromatograms Tell Us?
- Affinity Chromatography
- Ion Exchange Chromatography
- Gel Filtration Chromatography
- Cell Lysis and Fractionation
- Protein Dialysis, Desalting, and Concentration
Sample Quality Assessment: The Critical Bridge to Successful SPA Cryo-EM
Sample quality assessment represents a crucial checkpoint in the cryo-EM workflow, bridging the gap between protein purification and grid preparation. While traditional biochemical methods are essential, cryo-EM samples require additional specialized evaluation to ensure optimal structural analysis outcomes.
Biochemical Screening Methods
High-quality cryo-EM analysis demands rigorous sample characterization through multiple complementary techniques:
| Techniques | Description |
| SDS-PAGE and Western Blot Analysis | Pure and intact protein samples are essential for high-resolution structure determination, with SDS-PAGE providing crucial information about sample purity and potential degradation products that could complicate structural analysis. |
| Size Exclusion Chromatography | SEC profiles offer valuable insights into sample homogeneity and oligomeric state, helping identify optimal buffer conditions and storage parameters while detecting potential aggregation issues that could impact grid preparation. |
| Mass Spectrometry Analysis | Advanced MS techniques provide precise information about protein integrity, modifications, and complex composition, ensuring sample authenticity and helping identify potential heterogeneity that could affect 3D classification. |
Functional and Stability Assessment
- Thermal Stability Analysis
Techniques such as differential scanning fluorimetry (DSF) and thermal shift assays help evaluate protein stability under various conditions, guiding buffer optimization for cryo-EM grid preparation.
- Activity Measurements
Functional assays confirm that the purified protein maintains its native state and biological activity, essential for structural studies aiming to capture physiologically relevant conformations.
- Time-Course Stability Studies
Monitoring sample stability over time under various conditions helps predict behavior during grid preparation and storage, ensuring consistent sample quality throughout the experiment.
Advanced Characterization Methods
| Techniques | Description |
| Dynamic Light Scattering | DLS provides crucial information about sample monodispersity and potential aggregation, helping optimize conditions for successful grid preparation and high-quality data collection. |
| Negative Stain EM | A powerful pre-screening tool that provides initial structural insights and helps assess sample quality, particle distribution, and concentration before proceeding to costly cryo-EM analysis. |
| Native Mass Spectrometry | Offers insights into complex stoichiometry and stability under near-native conditions, helping identify optimal buffer conditions for structural studies. |
Quality Benchmarks and Decision Points
Sample Acceptance Criteria
- Purity level typically >95% by SDS-PAGE
- Monodisperse distribution in DLS
- Consistent activity measurements
- Stable SEC profiles over time
Critical Decision Making
Assessment results guide crucial decisions about:
- Proceeding to grid preparation
- Need for additional optimization
- Sample concentration adjustments
- Buffer modifications
Select Service
Cryo-EM Vitrification: A Critical Step for High-Resolution Structure Determination
The vitrification process represents a pivotal moment in cryo-EM sample preparation, where protein samples must be rapidly frozen to preserve their native state in a thin layer of amorphous ice. This process demands precise control and optimization to achieve optimal results for structural analysis.
Fundamentals of Cryo-EM Vitrification
Vitrification involves ultra-rapid cooling of aqueous samples to form amorphous ice, preventing the formation of crystalline ice that could damage biological structures. This process must occur at cooling rates exceeding 10^5 °C/second to achieve proper vitrification, resulting in samples embedded in electron-transparent vitreous ice.
Vitrification Equipment and Tools
Automated Plunge Freezers
Modern vitrification relies on automated systems (such as Vitrobot, Leica EM GP2, or Chameleon) that provide precise control over:
- Chamber temperature and humidity
- Blotting parameters
- Plunging speed and timing
- Environmental conditions
Essential Components
- High-quality EM grids (typically copper or gold)
- Liquid ethane/propane cooling system
- Humidity and temperature-controlled chamber
- Precision blotting mechanism
Critical Parameters for Successful Vitrification
| Sample Preparation |
|
| Grid Preparation |
|
| Vitrification Conditions |
|
Cryo EM Grid Screening and Optimization: The Final Quality Gate
Grid screening and optimization serve as critical quality control steps that bridge sample vitrification and high-resolution data collection. This evaluation process is essential for determining whether vitrified samples are suitable for structural analysis and for optimizing conditions to achieve the highest quality data possible.
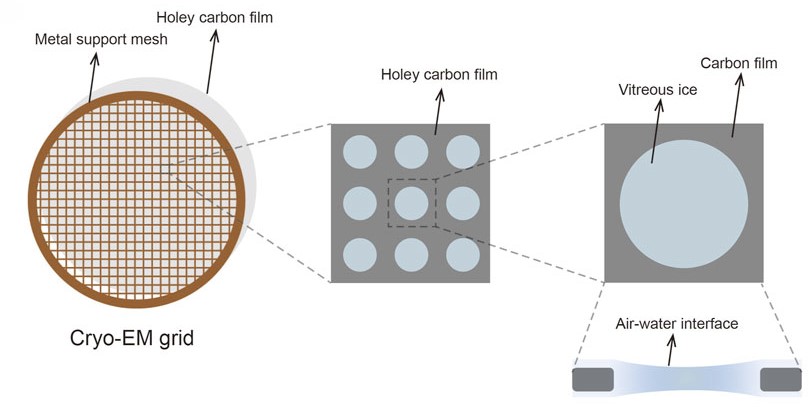 Figure 1. Cryo-EM grid composed of a metal support mesh and a holey carbon film designed to carry the sample solution. (Xu Y, et al., 2022)
Figure 1. Cryo-EM grid composed of a metal support mesh and a holey carbon film designed to carry the sample solution. (Xu Y, et al., 2022)
Cryo-EM Grid Types and Selection
The choice of grid type fundamentally impacts sample behavior and imaging quality. Holey carbon films remain the standard choice, while specialized supports like graphene oxide or gold foil grids offer advantages for specific applications. Selection criteria include particle size, sample characteristics, and desired resolution targets.
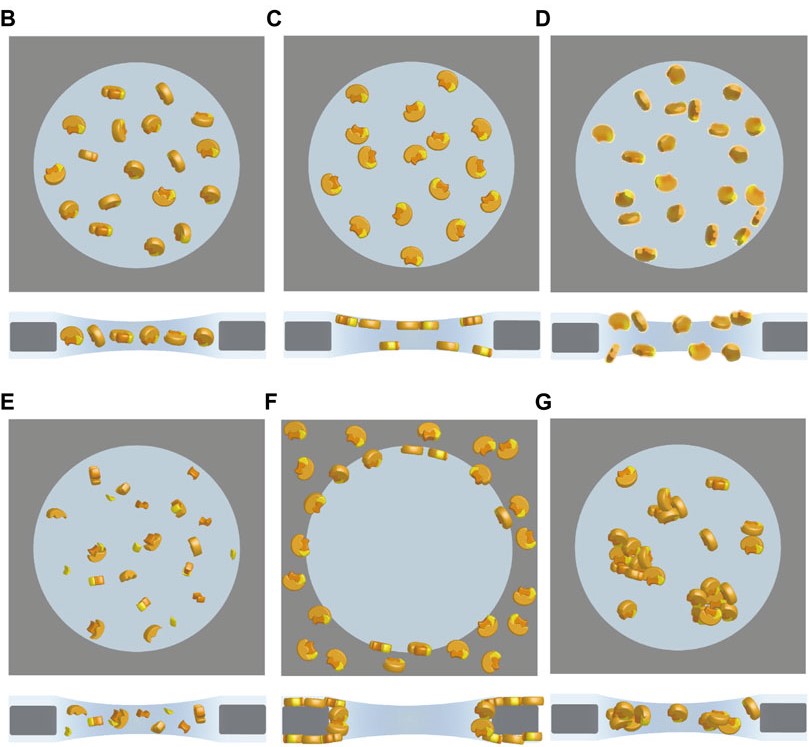 Figure 2. Cryo-specimens in single-particle cryo-EM frozen in vitreous ice for image acquisition. Different states of particles include ideal orientation (B), preferred orientation (C), denaturation (D), disassembly (E), adsorption on the carbon film (F), and aggregation (G). (Xu Y, et al., 2022)
Figure 2. Cryo-specimens in single-particle cryo-EM frozen in vitreous ice for image acquisition. Different states of particles include ideal orientation (B), preferred orientation (C), denaturation (D), disassembly (E), adsorption on the carbon film (F), and aggregation (G). (Xu Y, et al., 2022)
Initial Screening Process
Diagnostic screening using cryo-EM provides crucial qualitative assessment of sample suitability for structural analysis. This evaluation focuses on three key aspects: ice quality and thickness uniformity across the grid, particle distribution and concentration, and initial assessment of sample integrity and stability. These parameters directly influence the feasibility of obtaining high-quality 2D class averages and preliminary 3D reconstructions.
Optimization of Grid Conditions
Grid optimization involves systematic adjustment of multiple parameters:
- Surface treatment methods for optimal particle distribution
- Buffer composition refinement to enhance stability
- Sample application and blotting parameters for ideal ice thickness
- Environmental conditions during grid preparation
Quality Assessment and Decision Making
The screening process helps determine whether samples are suitable for high-resolution data collection. Initial low-magnification assessment provides crucial information about grid quality and usability. If promising, collection of a small dataset can verify the potential for high-resolution structure determination through 2D classification and preliminary 3D reconstruction, typically aiming for moderate resolution (>3Å) at this stage. Successful screening not only validates sample quality but also helps establish optimal imaging conditions for subsequent high-resolution data collection.
Successful single particle cryo-EM analysis relies heavily on meticulous sample preparation. Each step, from protein expression to grid screening, requires expertise, precision, and attention to detail. The quality of your final structural data is directly influenced by decisions made during this preparation process.
At Creative Biostructure, we offer single particle cryo-EM service backed by years of expertise and state-of-the-art facilities. Our experienced team specializes in optimizing each step of the sample preparation workflow to ensure the highest quality results for your structural biology projects. contact us for customized single particle cryo-EM solutions. We provide end-to-end support, from initial consultation to final structure determination, helping you achieve your research goals efficiently and effectively.
References
- Cheng Y, Grigorieff N, Penczek P A, et al. A primer to single-particle cryo-electron microscopy. Cell. 2015, 161(3): 438-449.
- Thompson R F, Walker M, Siebert C A, et al. An introduction to sample preparation and imaging by cryo-electron microscopy for structural biology. Methods. 2016, 100: 3-15.
- Passmore L A, Russo C J. Specimen preparation for high-resolution cryo-EM. Methods in Enzymology. 2016, 579: 51-86.
- Sader K, et al. Industrial cryo-EM facility setup and management. Acta Crystallographica Section D: Structural Biology. 2020, 76(4): 313-325.
- Xu Y, Dang S. Recent technical advances in sample preparation for single-particle cryo-EM. Frontiers in Molecular Biosciences. 2022, 9: 892459.

-1.jpg)