Algae Expression
The expression of proteins in algae has emerged as a cornerstone of biotechnology and synthetic biology, revolutionizing industries ranging from pharmaceuticals to sustainable food production. Algae, particularly microalgae, offer a distinct set of advantages over traditional protein expression systems such as bacteria, yeast, and mammalian cells. Their rapid growth, high productivity, and ability to thrive in diverse environments make them a compelling choice for producing recombinant proteins.
Creative Biostructure provides protein production services across various expression systems, delivering high-purity proteins with enhanced properties and comprehensive characterization data to support the success of your research, backed by proven expertise.
The Basics of Algae Protein Expression
Protein expression in algae involves the production of proteins from genetically modified algal cells, often using recombinant DNA technology. In this process, foreign genes—typically encoding specific proteins—are introduced into the genetic makeup of the algae. The resulting modified algae can then express these proteins under appropriate cultivation conditions. Algae, particularly species of Chlamydomonas reinhardtii, Chlorella vulgaris, and Scenedesmus obliquus, have attracted attention for their ability to produce high-quality proteins with favorable post-translational modifications, which is often a limiting factor in other expression systems.
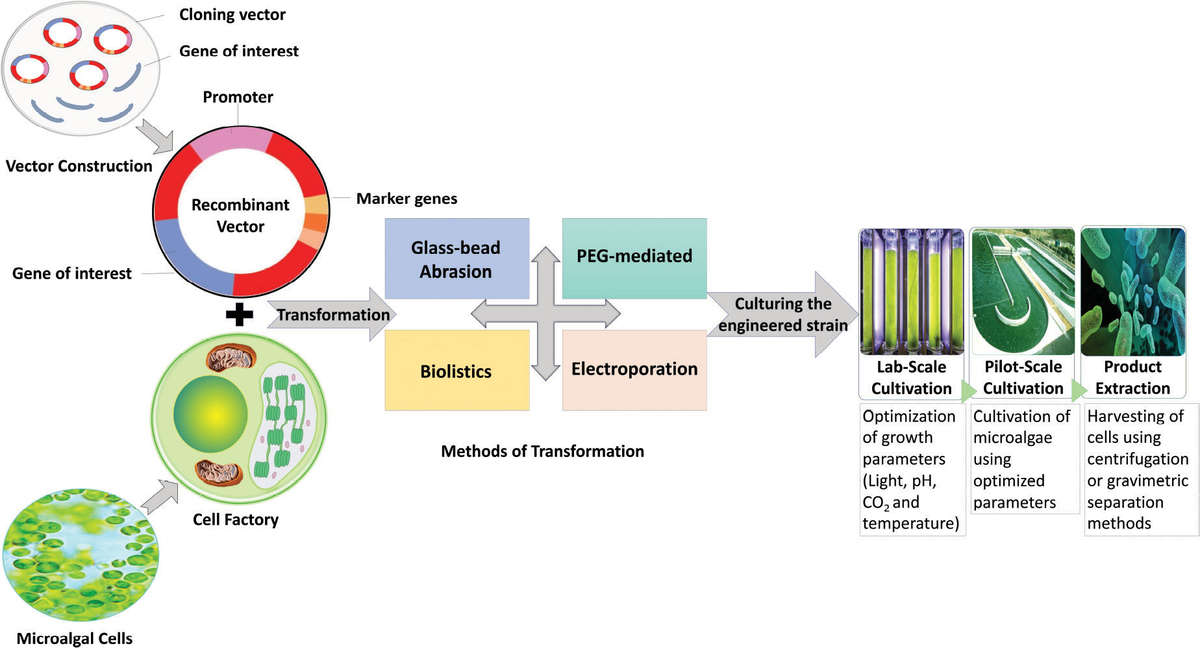 Figure 1. Process of algal protein expression. (Ahmad et al., 2022)
Figure 1. Process of algal protein expression. (Ahmad et al., 2022)
Types of Algae Used for Protein Expression
Microalgae, primarily green algae, are the most studied group for protein expression. Their unicellular nature allows for easy genetic manipulation and large-scale cultivation. Among them, Chlamydomonas reinhardtii is particularly prominent due to its well-characterized genome and the established methods for genetic transformation, such as particle bombardment and electroporation. Another widely used species is Chlorella vulgaris, which is known for its robust growth and the ability to produce significant biomass in controlled environments.
Macroalgae, or seaweeds, although less commonly used for protein expression, are also being explored for their large biomass production and ease of harvesting. Species such as Porphyra (nori) and Fucus (bladderwrack) hold promise for future recombinant protein expression, particularly in the context of sustainable agriculture and marine biotechnology.
 Figure 2. Examples of algae used for protein expression. Left: Microscopic view of the unicellular alga Chlamydomonas reinhardtii. Right: Photograph of the macroalgae Fucus serratus.
Figure 2. Examples of algae used for protein expression. Left: Microscopic view of the unicellular alga Chlamydomonas reinhardtii. Right: Photograph of the macroalgae Fucus serratus.
Methodologies for Protein Expression in Algae
The core methodology for algal protein expression revolves around genetic engineering. Over the years, a variety of transformation techniques have been developed to introduce exogenous genes into algal cells. These methods can be broadly classified into physical, chemical, and biological techniques.
Agrobacterium-Mediated Transformation
Agrobacterium tumefaciens, a bacterium known for its ability to transfer DNA into plant cells, has been successfully adapted for use in algae. This method takes advantage of the bacterium's natural genetic transformation mechanisms. For algae, this technique provides a relatively efficient way to integrate foreign genes into the algal genome. It is often used in conjunction with a selection marker to ensure only transformed cells proliferate.
Biolistic or Particle Bombardment
This method involves the physical delivery of DNA-coated microprojectiles (typically gold or tungsten) into algal cells via high-pressure acceleration. Although labor intensive, biolistic transformation has the advantage of being applicable to a wide range of algal species. It is particularly useful when dealing with species that are difficult to transform by other means.
Electroporation
Electroporation involves applying an electric field to increase the permeability of the algal cell membrane, allowing the introduction of DNA molecules into the cell. This technique is particularly effective for species that have a tough outer membrane, such as Chlorella and Scenedesmus. Electroporation has the added benefit of being a scalable method, making it suitable for large-scale protein production.
Viral Vectors
In some cases, algae can be engineered to express proteins using viral vectors. Algal viruses, such as those from the family Phycodnaviridae, are being studied for their ability to carry genetic material into algal cells. The advantage of this method lies in its efficiency, though it remains less developed compared to other methods.
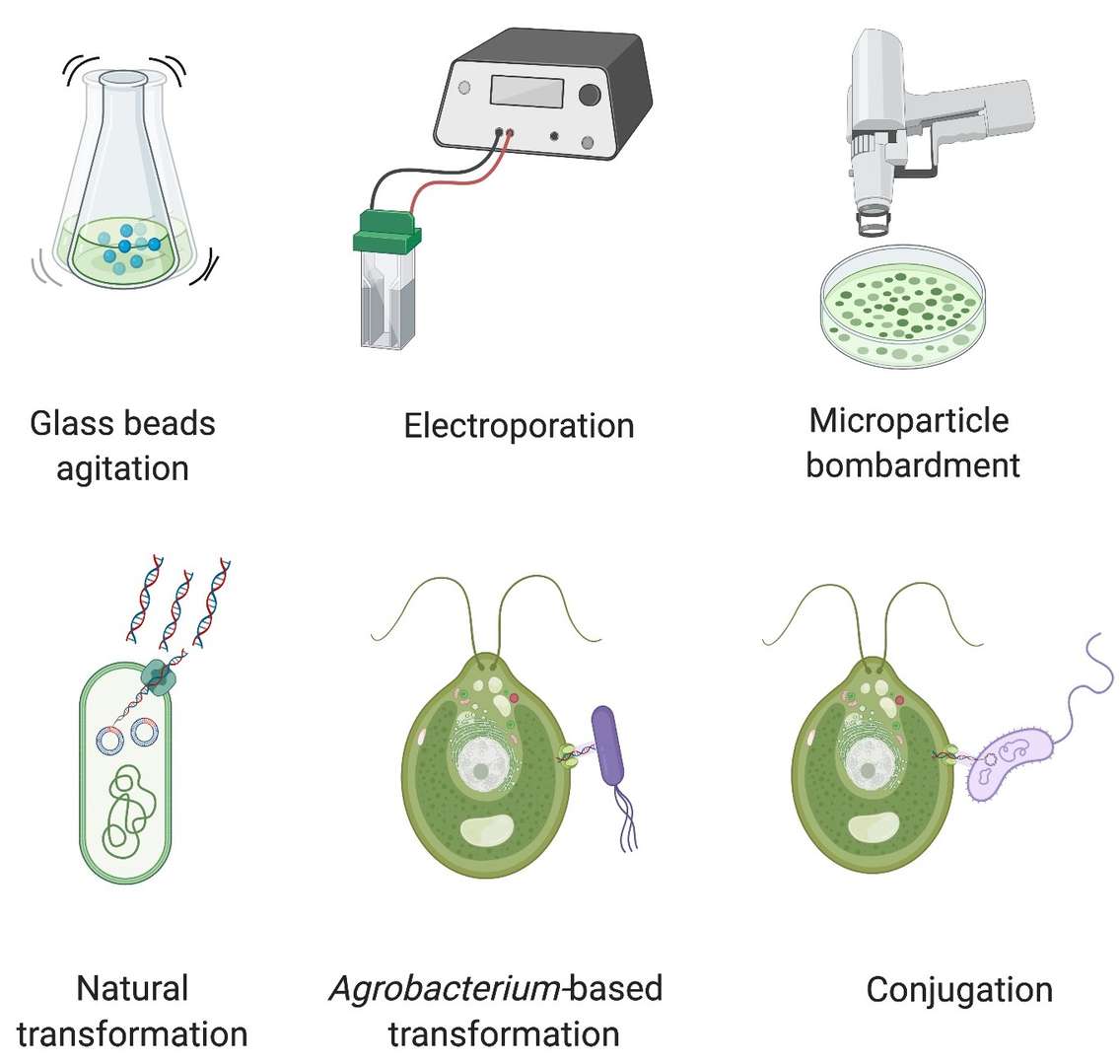 Figure 3. Transformation methods in algae. (Adapted from Gutiérrez and Lauersen, 2021)
Figure 3. Transformation methods in algae. (Adapted from Gutiérrez and Lauersen, 2021)
Once transformation is successfully achieved, the recombinant algae are cultivated under optimized growth conditions. The algae can be cultured in photobioreactors, open ponds, or even under stress conditions that induce protein expression. Light, temperature, nutrient availability, and CO2 concentration are all variables that can influence the expression levels of the recombinant proteins.
Protein Purification from Algae
After the recombinant proteins are expressed in algae, the next challenge is their extraction and purification (Protein Purification). Algal cells are often harvested, disrupted, and the proteins are isolated using methods similar to those used in other systems, such as chromatography and centrifugation. However, the presence of polysaccharides and lipids in algal cells can complicate the purification process. To overcome these challenges, strategies such as osmotic shock, enzyme treatments, and detergent-based extraction are often used.
A major advantage of using algae for protein production is their ability to secrete proteins into the culture medium. This simplifies the purification process and reduces the need for cell lysis, a step that can lead to contamination of the protein product.
Applications of Algae Protein Expression
The potential applications of algal protein expression are vast and varied, spanning multiple industries and scientific disciplines. Some of the most promising applications include:
Pharmaceuticals and Vaccines
Algae-based systems are being explored for the production of pharmaceutical proteins, including monoclonal antibodies, therapeutic enzymes, and even vaccines. One notable example is the use of Chlamydomonas for the production of antigens used in vaccines. The ability of algae to perform post-translational modifications such as glycosylation makes them a viable alternative to mammalian systems, particularly in cases where traditional mammalian cell culture systems are too costly or inefficient.
Food and Feed Industry
Algae proteins are increasingly recognized as a sustainable and nutritious alternative to animal-based proteins. Algae such as Chlorella and Spirulina are already used as dietary supplements and are being considered for use in plant-based meat alternatives. In the feed industry, algal proteins are gaining traction as a high-protein, low-cost supplement for livestock and aquaculture. In addition, algae proteins can be a key component in synbiotic blends to improve animal gut health.
Biofuels and Renewable Energy
In the context of biofuels, algae are being engineered to produce proteins that enhance lipid production or contribute to biofuel synthesis. Additionally, proteins involved in metabolic pathways that promote the conversion of sugars into biofuels are of significant interest. Algae protein expression systems could help optimize biofuel yields, making them a more sustainable energy source.
Industrial Enzymes
Algae have been successfully engineered to express industrially relevant enzymes, including those used in biofuels, textiles, food processing and waste treatment. Enzyme production in algae is attractive because of the lower costs associated with algae cultivation compared to more traditional systems such as bacteria or fungi.
Bioremediation
Algae are naturally adept at absorbing heavy metals and other contaminants from water. Genetically engineered algae could be used to enhance this ability, creating "biofactories" capable of producing proteins involved in detoxifying pollutants. This opens the door to environmentally friendly solutions for environmental remediation.
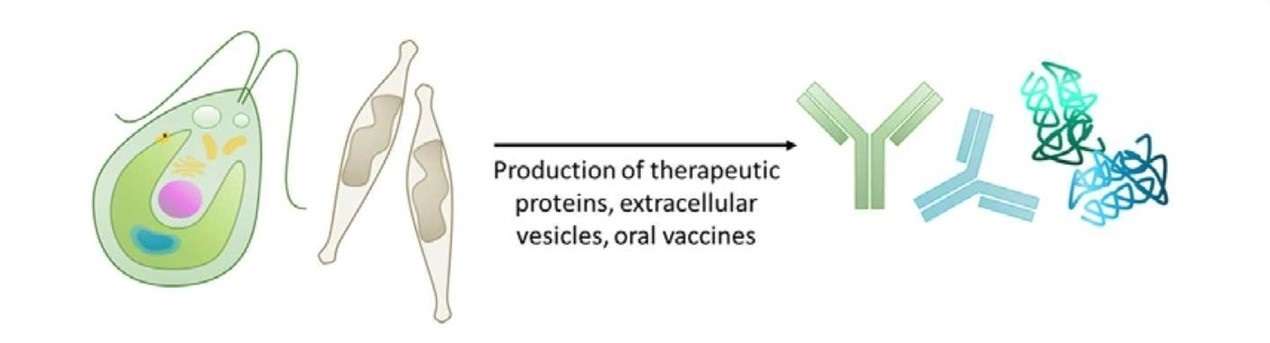 Figure 4. Production of recombinant and therapeutic proteins in microalgae. (Adapted from Banerjee and Ward, 2022)
Figure 4. Production of recombinant and therapeutic proteins in microalgae. (Adapted from Banerjee and Ward, 2022)
Algae protein expression holds immense promise as a sustainable, cost-effective, and versatile platform for producing a wide array of valuable proteins. From pharmaceuticals and vaccines to biofuels and industrial enzymes, the potential applications are vast.
Creative Biostructure delivers tailored solutions across major expression systems, ensuring your research or industrial objectives are met with precision and efficiency. Contact us today to enhance your protein expression capabilities!
References
- Ahmad N, Mehmood MA, Malik S. Recombinant protein production in microalgae: emerging trends. PPL. 2020;27(2):105-110.
- Banerjee A, Ward V. Production of recombinant and therapeutic proteins in microalgae. Current Opinion in Biotechnology. 2022;78:102784.
- Gutiérrez S, Lauersen KJ. Gene delivery technologies with applications in microalgal genetic engineering. Biology. 2021;10(4):265. doi:10.3390/biology10040265
