Structural Research of Type VI Secretion Systems
Bacterial pathogens use a variety of mechanisms to invade mammalian hosts, damage tissue sites, and evade the immune system. The type VI secretion system (T6SS), widespread in Gram-negative bacteria, is a transmembrane complex. It is thought to be a secretion system for virulence-effector proteins that specifically target eukaryotic host cells. In addition, the T6SS regulates bacterial interactions and competition, indirectly promoting host pathogenesis. In recent years, with the gradual maturation of cryo-electron microscopy technology, researchers have taken an important step in the resolution of the T6SS atomic model, which is essential for exploring the action mechanism of pathogenic bacteria.
Structural basis and action mechanism of T6SS
T6SS uses a spring-like mechanism to inject effectors into target cells. It is assembled from three main components, the membrane complex spanning the inner and outer membranes (TssJ、TssL、TssM), the substrate (TssE、TssF、TssG、TssK、VgrG、PAAR), and the sheath polymer (Hcp、TssB、TssC). The injection device consists of a substrate on which a retractable tail tube is constructed, and the inner tube, topped with a spiny complex, is pushed out of the cell through the contraction of the sheath. The injection system is anchored to the cell membrane and orientated towards the outside cell via the transmembrane complex. T6SS-delivered effectors loaded within the inner tube or on the spiny complex can target prokaryotic or eukaryotic cells.
Structural analysis of the Hcp5-VgrG-PAAR complex
B. fragilis NCTC 9343 is a representative strain of B. fragilis with a functional T6SS GA3 motif. To better understand the characterization of the core effector cargo delivery system in T6SS GA3, the researchers provided cryo-electron microscopy structures of the complete Hcp5-VgrG-PAAR complex. Before processing, all particles were binned according to a 2D classification scheme. The cryoelectron microscopy dataset underwent several rounds of refinement to a final resolution of 2.73 Å. The structure shows that the Hcp5-VgrG-PAAR symmetric complex assembles into a needle-like structure containing the full-length hexameric Hcp, trimeric VgrG, and the N-terminal structural domain of PAAR.
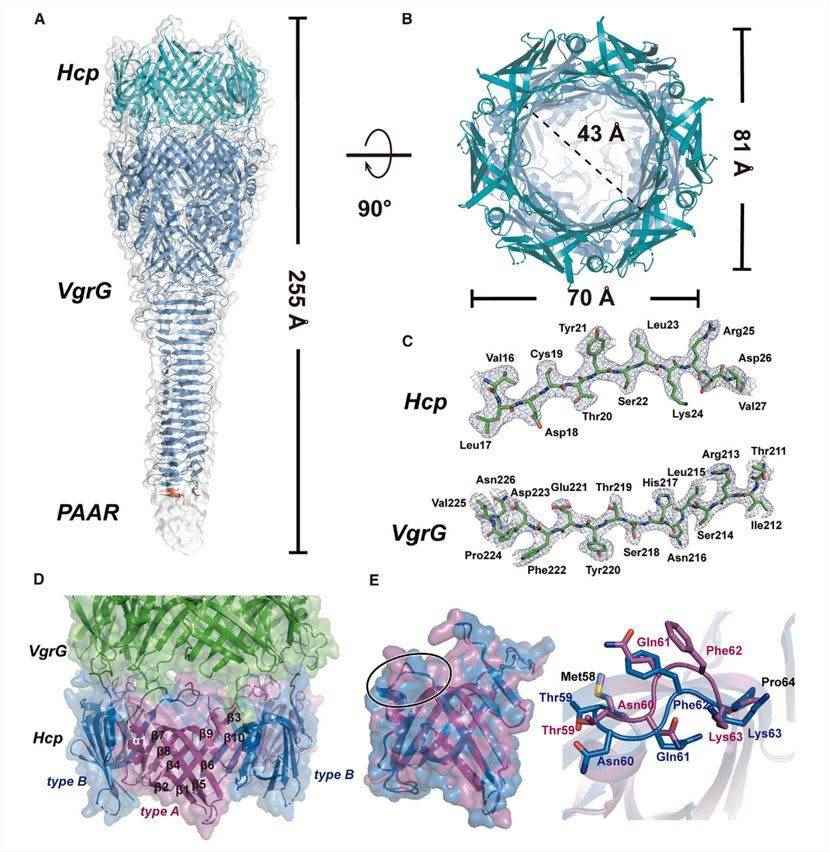 Figure 1. Cryo-electron microscopy structure of the Hcp-VgrG-PAAR complex. (He W, et al., 2023)
Figure 1. Cryo-electron microscopy structure of the Hcp-VgrG-PAAR complex. (He W, et al., 2023)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Type VI secretion system membrane core complex | Escherichia coli 55989 | Cryo-EM single particle analysis | 4 Å | 6IXH |
| Type VI membrane complex | Escherichia coli | Cryo-EM single particle analysis | 4.6 Å | 6HS7 |
| The Tle1 effector bound to the VgrG spike from the Type 6 secretion system | Escherichia coli | Cryo-EM single particle analysis | 2.6 Å | 6SJL |
| TssL | Vibrio cholerae | X-ray diffraction | 1.499 Å | 4V3I |
| VgrG1 | Pseudomonas protegens Pf-5 | Cryo-EM single particle analysis | 3.3 Å | 7Q5P |
| The VgrG spike | Escherichia coli | Cryo-EM single particle analysis | 2.3 Å | 6SK0 |
| VgrG1, the central spike of the type VI secretion system | Pseudomonas aeruginosa PAO1 | X-ray diffraction | 3.322 Å | 4MTK |
| The C-terminal region of VgrG1 | Escherichia coli O157:H7 | X-ray diffraction | 1.95 Å | 3WIT |
| The Tle hydrolase bound to the TTR domain of the VgrG spike of the Type 6 secretion system | Escherichia coli | Cryo-EM single particle analysis | 2.6 Å | 6SKI |
| T6SS SciP/TssL | Escherichia coli 042 | X-ray diffraction | 2.63 Å | 3U66 |
| Lip, a membrane-bound component of the Type VI Secretion System. | Serratia marcescens | X-ray diffraction | 1.92 Å | 4A1R |
| Effector domain of VgrG2b | Pseudomonas aeruginosa PAO1 | X-ray diffraction | 3.2 Å | 6H56 |
| Atomic model of the VipA/VipB/Hcp | Vibrio cholerae | Cryo-EM single particle analysis | 3.7 Å | 5MXN |
| T6SS protein TssM C-terminal domain (869-1107) | Escherichia coli | X-ray diffraction | 2.24 Å | 4Y7O |
| T6SS protein TssM C-terminal domain (869-1107) | Escherichia coli 2-156-04_S3_C3 | X-ray diffraction | 1.51 Å | 4Y7L |
| T6SS protein TssM C-terminal domain (835-1129) | Escherichia coli 2-156-04_S3_C3 | X-ray diffraction | 1.92 Å | 4Y7M |
| The type VI secretion system TssK-TssF-TssG baseplate subcomplex | Escherichia coli 042 | Cryo-EM single particle analysis | 3.7 Å | 6N38 |
| VgrG1 in the Type VI secretion "pre-firing" VgrG1-Tse6-EagT6-EF-Tu-Tsi6 complex | Pseudomonas aeruginosa PAO1 | Cryo-EM single particle analysis | 4.2 Å | 6H3L |
| The baseplate complex from the type VI secretion system | Escherichia coli | Cryo-EM single particle analysis | 4.7 Å | 6GJ1 |
| The baseplate complex from the type VI secretion system | Escherichia coli | Cryo-EM single particle analysis | 4.3 Å | 6GIY |
| VgrG1, the needle tip of the type VI secretion system | Pseudomonas aeruginosa PAO1 | X-ray diffraction | 2 Å | 4UHV |
| VgrG1 in the type VI secretion VgrG1-Tse6-EF-Tu complex | Pseudomonas aeruginosa PAO1 | X-ray diffraction | 3.25 Å | 6H3N |
| Type VI secretion system cargo delivery vehicle Hcp-VgrG-PAAR | Bacteroides fragilis | Cryo-EM single particle analysis | 2.8 Å | 8GRA |
| T6SS lipoprotein TssJ1 | Pseudomonas aeruginosa PAO1 | X-ray diffraction | 1.4 Å | 3ZHN |
| Truncated derivative of the C-terminal domain of the TssA component of the type VI secretion system | Burkholderia cenocepacia H111 | X-ray diffraction | 2.35 Å | 6H8E |
| C-terminal domain of TssA protein from T6SS | Escherichia coli | Cryo-EM single particle analysis | 3.2 Å | 6RJU |
| The C-terminal domain of the TssA component of the type VI secretion system | Burkholderia cenocepacia H111 | X-ray diffraction | 3.08 Å | 6HS6 |
| Hcp1 protein | Escherichia coli | X-ray diffraction | 155 Å | 4W64 |
| T6SS Hcp protein | Chromobacterium haemolyticum | X-ray diffraction | 3.3 Å | 7FCF |
| The extended type VI secretion system sheath | Myxococcus xanthus DK 1622 | Cryo-EM single particle analysis | 24 Å | 5URW |
Table 1. Structural research of the type VI secretion systems.
Creative Biostructure provides cutting-edge X-ray crystallography, cryo-electron microscopy (cryo-EM), and NMR spectroscopy services to support structural biology research. Our team of experienced scientists has extensive expertise in high-resolution resolving of complex macromolecular structures including type VI secretion systems. In addition, our equipment is equipped with the latest sample preparation, data acquisition, and image processing instruments.
Our strength lies in our commitment to quality, speed, and cost-effectiveness. We combine cutting-edge technology, advanced software, and state-of-the-art instrumentation to deliver high-quality structural data in a timely and cost-effective manner. Our team works closely with clients throughout the project, providing detailed reports and consultation to help them understand the structural insights gained from our analyses. If you are interested in our services, please feel free to contact us to learn more details.
References
- He W, et al. Structure and assembly of type VI secretion system cargo delivery vehicle. Cell Rep. 2023. 42(7): 112781.
- Nazarov S, et al. Cryo-EM reconstruction of Type VI secretion system baseplate and sheath distal end. EMBO J. 2018. 37(4): e97103.
- Cherrak Y, et al. Structure and Activity of the Type VI Secretion System. Microbiol Spectr. 2019. 7(4): 10.1128/microbiolspec. PSIB-0031-2019.
