Structural Research of Glucosyltransferases
Glucosyltransferases play a vital role in the process of N-glycosylation, a post-translational modification essential for the proper folding, stability, and function of glycoproteins in eukaryotic cells. Understanding the structural characteristics and mechanisms of these enzymes is crucial for unraveling the intricacies of N-glycosylation and its implications in various biological processes and diseases. N-Glycosylation involves the addition of glycan chains to specific asparagine residues on nascent glycoproteins. Glucosyltransferases are responsible for catalyzing the transfer of glucose moieties onto the glycan structure. This process occurs primarily in the lumen of the endoplasmic reticulum (ER) and is crucial for the quality control and maturation of glycoproteins.
Understanding the Architecture of Glucosyltransferases
Researchers have made significant progress in elucidating the structural characteristics of glucosyltransferases. Cryo-electron microscopy (cryo-EM) has emerged as a powerful technique for visualizing these enzymes at high resolution. A notable study presented the cryo-EM structure of yeast ALG6, a glucosyltransferase involved in N-glycosylation, at a resolution of 3.0 Å. This breakthrough revealed a previously undescribed transmembrane protein fold, providing valuable insights into the architecture of this crucial enzyme.
Modular Architecture of Glucosyltransferases
Further investigations into the structure of glucosyltransferases have unveiled their modular architecture. Studies suggest that these enzymes, including those belonging to the C-superfamily (GT-Cs), consist of a conserved module and a variable module. The conserved module plays a fundamental role in the catalytic mechanism, while the variable module contributes to substrate recognition and specificity. This modular architecture allows for the diverse functions and activities exhibited by different glucosyltransferases.
Active Site Insights
By employing cryo-EM in combination with synthetic analogues and enzymatic glycan extension, researchers have gained valuable insights into the active sites of glucosyltransferases. Another cryo-EM study, successfully visualized ALG6 bound to an analogue of dolichylphosphate-glucose at a resolution of 3.9 Å. This detailed structure provided a clearer understanding of the catalytic mechanism and identified a conserved catalytic aspartate residue that likely acts as a general base in the enzymatic reaction.
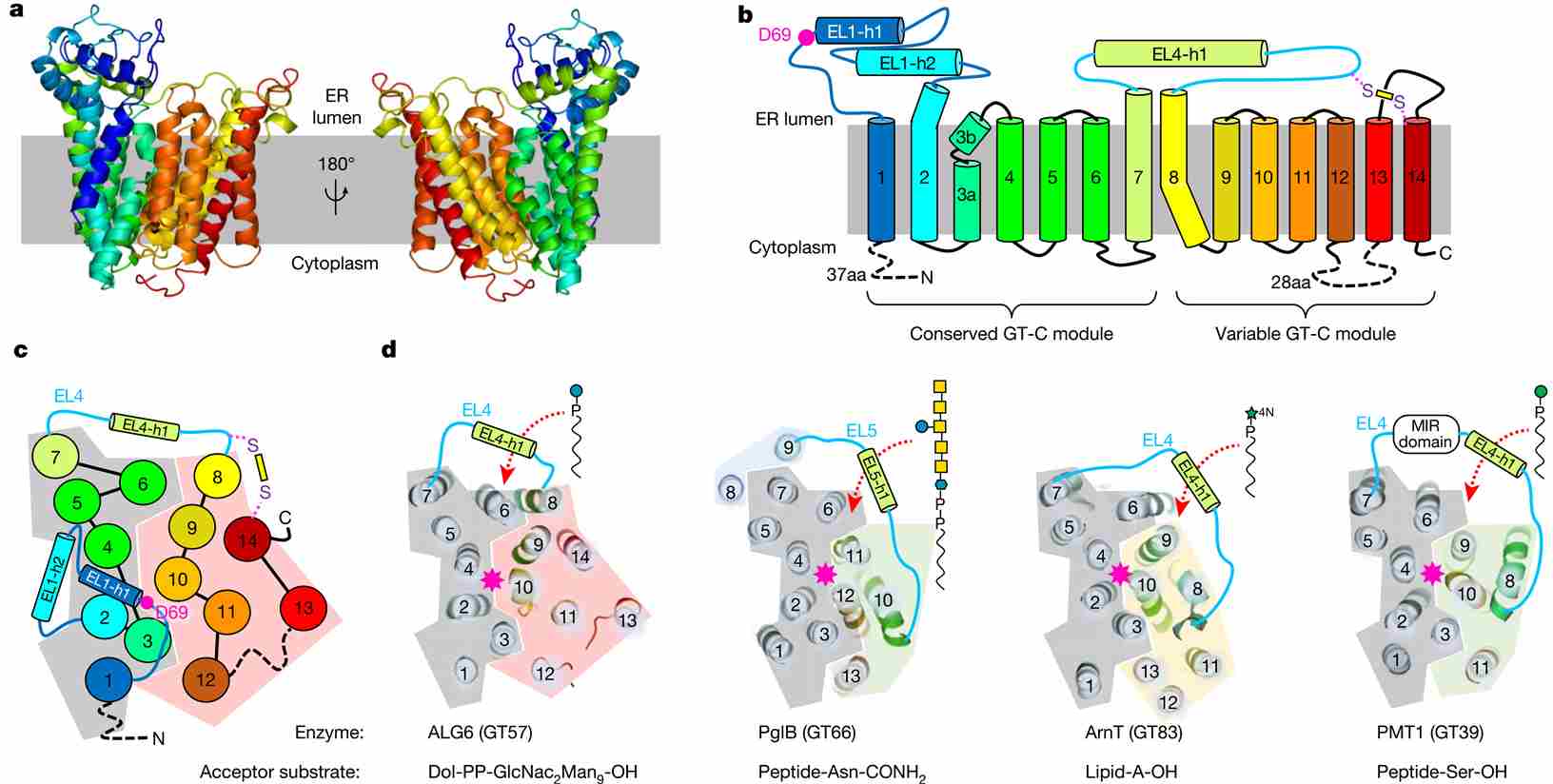 Figure 1. ALG6 structure and GT-C architecture. (Bloch J S, et al., 2020)
Figure 1. ALG6 structure and GT-C architecture. (Bloch J S, et al., 2020)
| Protein | Organism | Method | Resolution | PDB Entry ID |
|---|---|---|---|---|
| ALG6 glucosyltransferase in nanodiscs (expressed in Spodoptera frugiperda) | Saccharomyces cerevisiae, synthetic construct | Cryo-EM single particle analysis | 3.00 Å | 6SNI |
Table 1. Structural Research of Glucosyltransferases.
Creative Biostructure, a leading company specializing in structural biology, offers state-of-the-art structural analysis services for glucosyltransferases and other biomolecules. We stand out in the field of structural biology due to our unmatched expertise, state-of-the-art facilities, and commitment to delivering high-quality results. Our team of scientists combines extensive knowledge, technical proficiency, and a passion for scientific discovery to provide exceptional structural analysis services. With a track record of successful collaborations and satisfied clients, we are the trusted partner for elucidating the structural mysteries of glucosyltransferases and other biomolecules. Contact us today to learn more about our structural analysis techniques and how we can help you advance your research.
Reference
- Bloch J S, et al. Structure and mechanism of the ER-based glucosyltransferase ALG6. Nature. 2020, 579(7799): 443-447.
