Exosomes for mRNA Delivery
In past research, therapeutic messenger RNA (mRNA) has emerged as a promising new protein replacement therapy drug. Adding modified nucleotides to synthetic mRNA maximizes protein expression and reduces adverse immunogenicity. Despite these impressive advances, mRNA therapeutics are still limited by the need to develop safe and effective vectors to protect the integrity of the mRNA for in vivo application. Recently, exosomes have been developed as prime candidates for drug delivery vehicles. Exosomes are nanoscale extracellular vesicles, typically between 30-150nm, secreted by virtually all human cell types. Due to the inherent biocompatibility, ability to cross physiological barriers and low immunogenicity, exosomes have emerged as promising carriers for nucleic acid-based therapies.
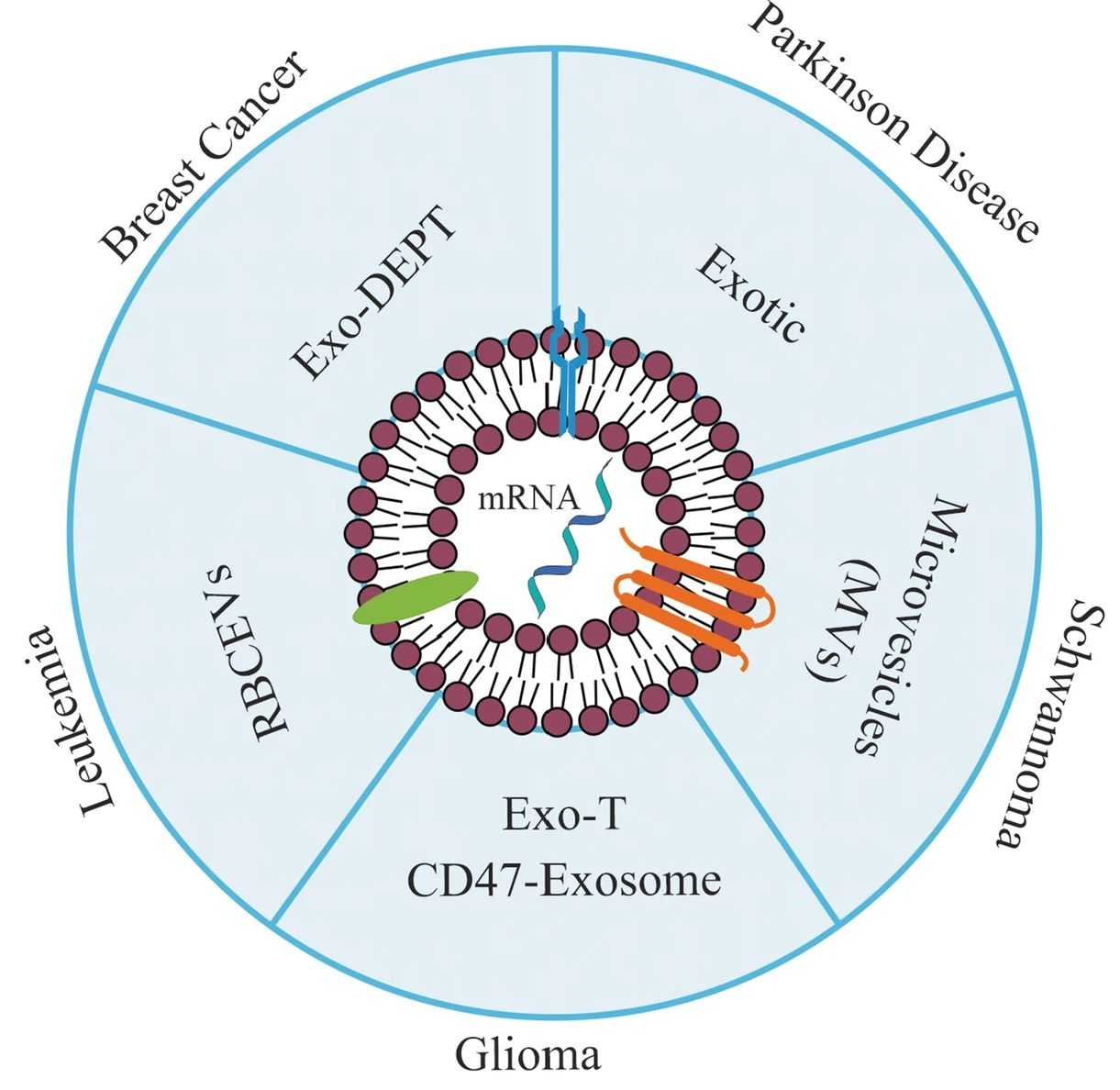 Figure 1. Exosome-mediated mRNA delivery for personalized medicine. (L Volpini, et al., 2023)
Figure 1. Exosome-mediated mRNA delivery for personalized medicine. (L Volpini, et al., 2023)
What are the Barriers to mRNA Therapy?
Clinical translation of mRNA-based therapies requires safe and effective delivery systems. Although considerable progress has been made in the development of mRNA delivery systems, many challenges remain, such as dose-limiting toxicity and specific delivery to extrahepatic tissues. One of the most critical barriers to the use of mRNA as a therapeutic agent is the immunogenicity of in vitro transcribed mRNA (IVT). Many cells recognize single-stranded RNAs (SSRs) and double-stranded RNAs (DSRs) via pattern recognition receptors (PRRs) and induce gene expression of pro-inflammatory cytokines (PICs) and type I interferons (IFNs). Systemic delivery of unpurified IVT mRNA stimulates an immune response that induces (PIC) and (IFN) expression. In addition, IVT mRNA is recognized by the retinoic acid-inducible gene I (RIG-I)-like receptor (RLR), a cytoplasmic RNA deconjugating enzyme that plays a major role in innate immune and non-immune (epithelial) cells.
Why Deliver mRNA by Exosomes?
Despite recent advances in the development of nanomaterials for the delivery of cancer therapeutics, the realization of an ideal drug delivery system requires the avoidance of toxicity, immunogenicity, and other side effects. To overcome these obstacles, exosomes are considered as an efficient drug delivery device. Due to the inherent nanoscale size and natural cellular products, exosomes can evade degradation by phagocytes and are naturally stable. Research has shown that exosomes can cross the blood-brain barrier (BBB) with intrinsic targeting properties based on their composition.
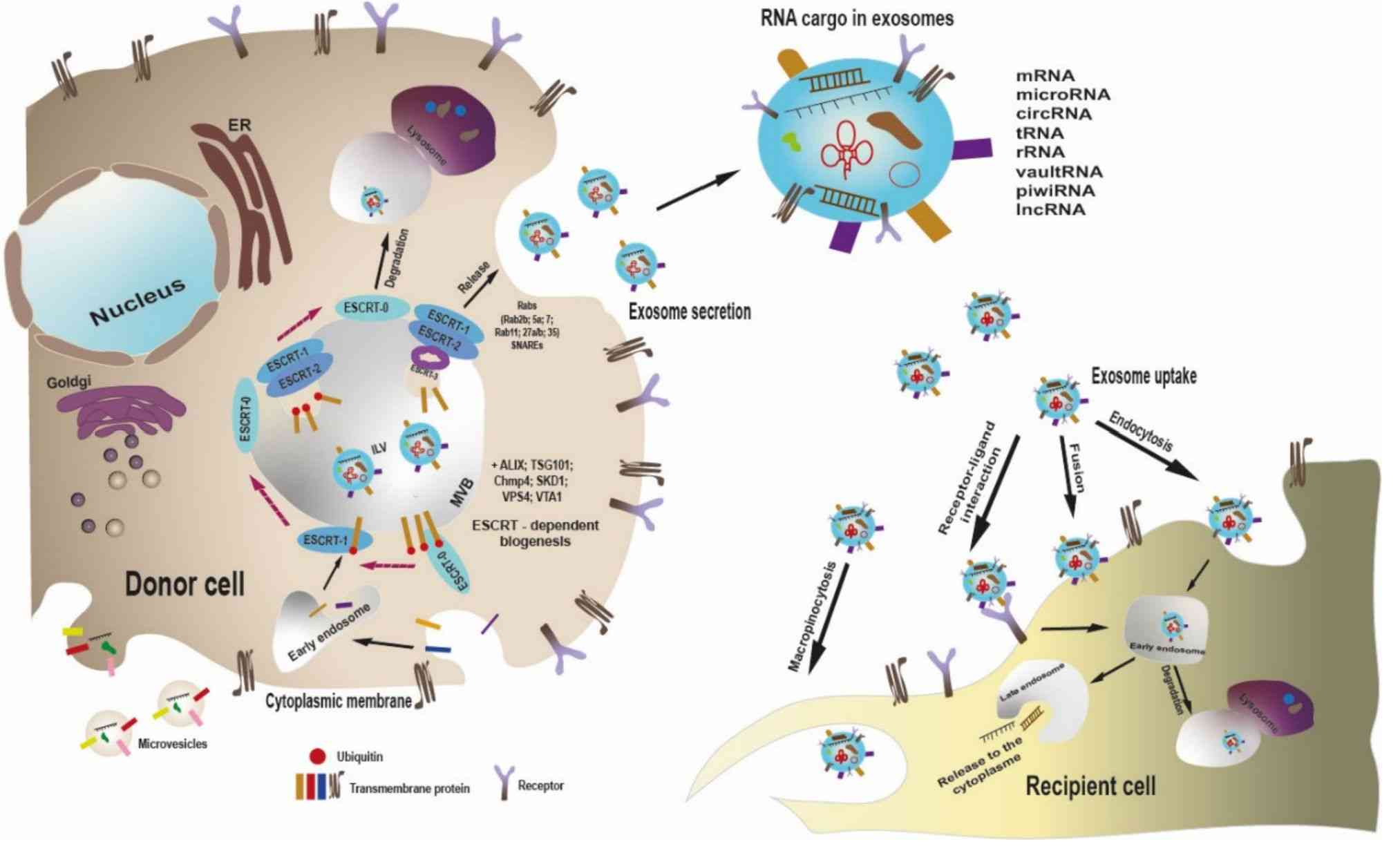 Figure 2. Scheme of exosome biogenesis, release, and uptake by recipient cell. (L Volpini, et al., 2023)
Figure 2. Scheme of exosome biogenesis, release, and uptake by recipient cell. (L Volpini, et al., 2023)
How to Load mRNA into Exosomes?
Strategies for loading mRNA into exosomes can be divided into two broad categories, pre-loading and post-loading. Pre-loading methods rely on producing cells to package mRNA cargo into exosomes during biogenesis. During endogenous loading, the mRNA-encoded proteins are also packaged into exosomes simultaneously. Preloading methods can be further categorized as passive and active. Post-loading methods are also referred to as post-isolation or exogenous loading. In this case, exogenous mRNA is loaded into isolated exosomes by electroporation or chemical transfection reagents.
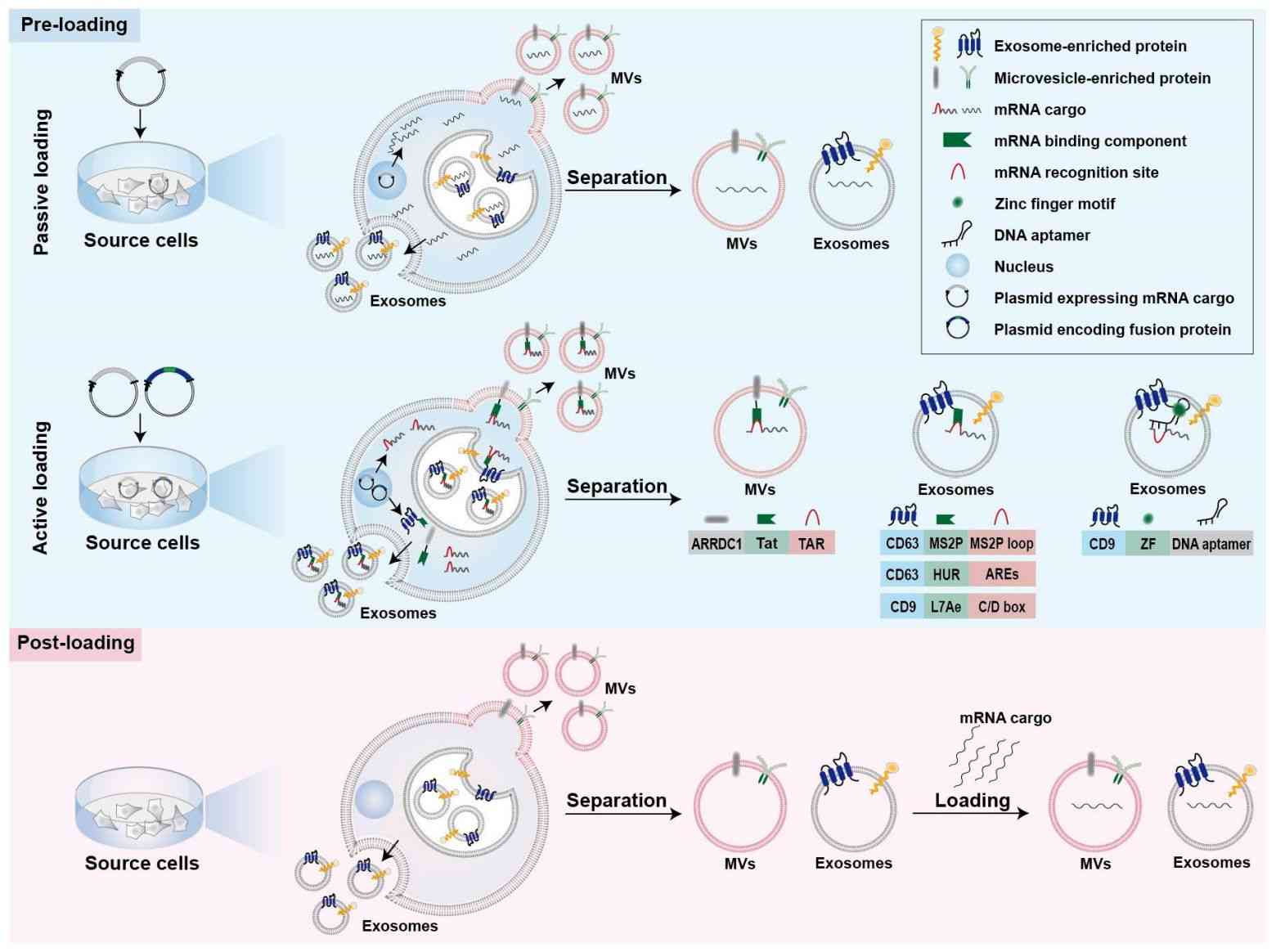 Figure 3. Strategies for mRNA loading into exosomes. (L Volpini, et al., 2023)
Figure 3. Strategies for mRNA loading into exosomes. (L Volpini, et al., 2023)
Research Cases of mRNA Delivery by Exosomes
The success of mRNA therapeutics depends largely on the availability of delivery systems that can safely, efficiently, and consistently convert genetic material into functional proteins. Researchers encapsulated mRNA encoding extracellular matrix alpha1 collagen (COL1A1) through exosomes produced from human dermal fibroblasts, which induced the formation of collagen grafts and reduced wrinkle formation in collagen-deficient dermal tissue. In addition, research has found that intradermal delivery of mRNA-containing exosomes via microneedle arrays prolongs the synthesis and replacement of collagen more uniformly in the animal dermis. Intradermal delivery of exosome-based COL1A1 mRNA may become an effective protein replacement therapy for the treatment of photoaged skin.
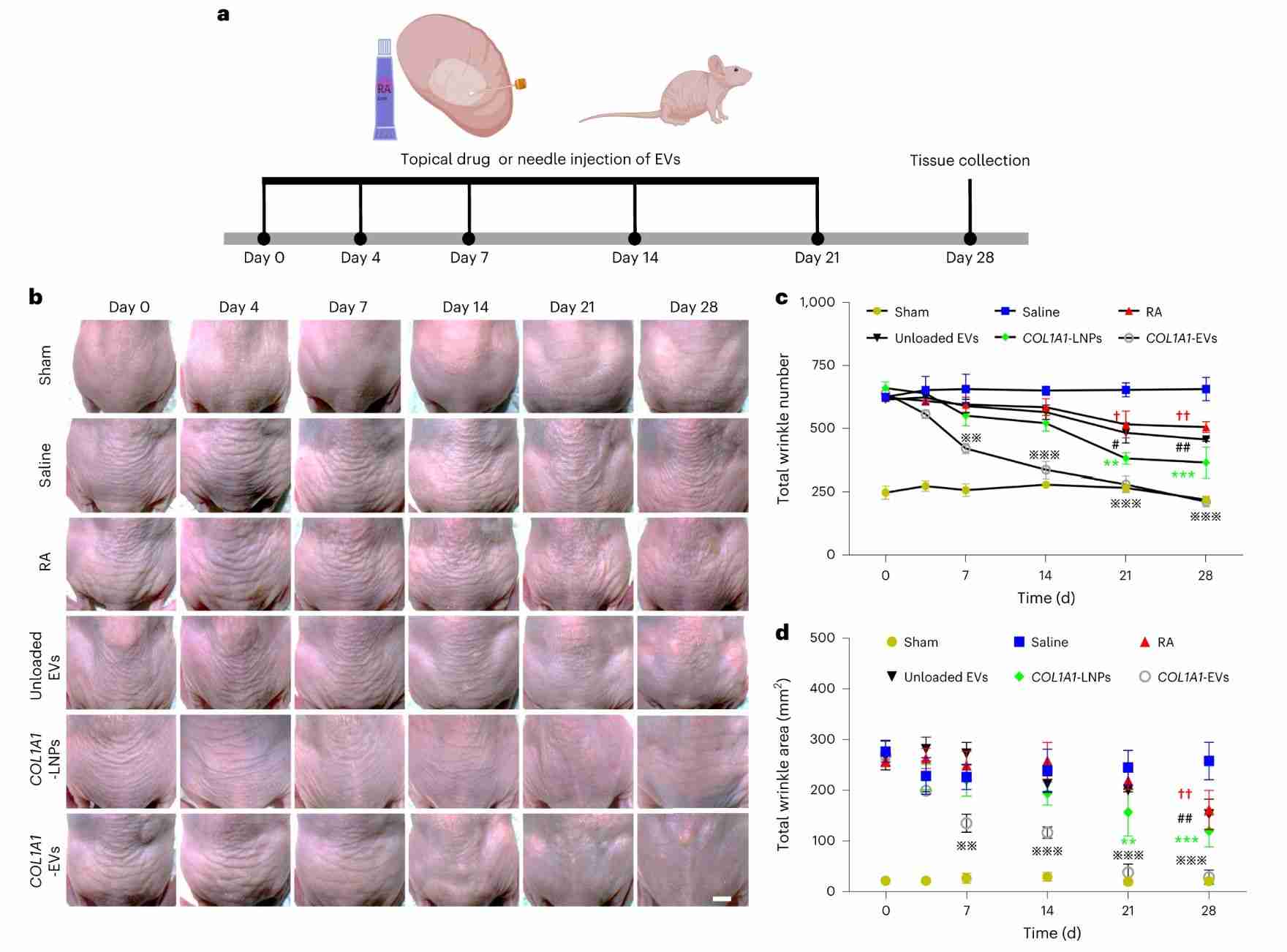 Figure 4. COL1A1-EV mRNA delivery reduces dermal wrinkles in a UV-irradiation photoaging model. (L Volpini, et al., 2023)
Figure 4. COL1A1-EV mRNA delivery reduces dermal wrinkles in a UV-irradiation photoaging model. (L Volpini, et al., 2023)
Respiratory diseases are one of the leading causes of morbidity and mortality worldwide. mRNA lipid nanoparticle (LNP) vaccines have been developed, but their intramuscular delivery limits pulmonary bioavailability. Inhaled nanoparticle therapies provide local drug delivery that minimizes targeted adverse effects. Lung-derived exosomes (Lung-Exo) offer a biological nanoparticle alternative naturally optimized for mRNA translation and delivery to lung cells. Research has found that Lung-Exo exhibits superior mRNA and protein delivery following nebulized administration. This suggests that inhaled Lung-Exo can deliver mRNA and protein drugs with enhanced lung bioavailability and therapeutic efficacy.
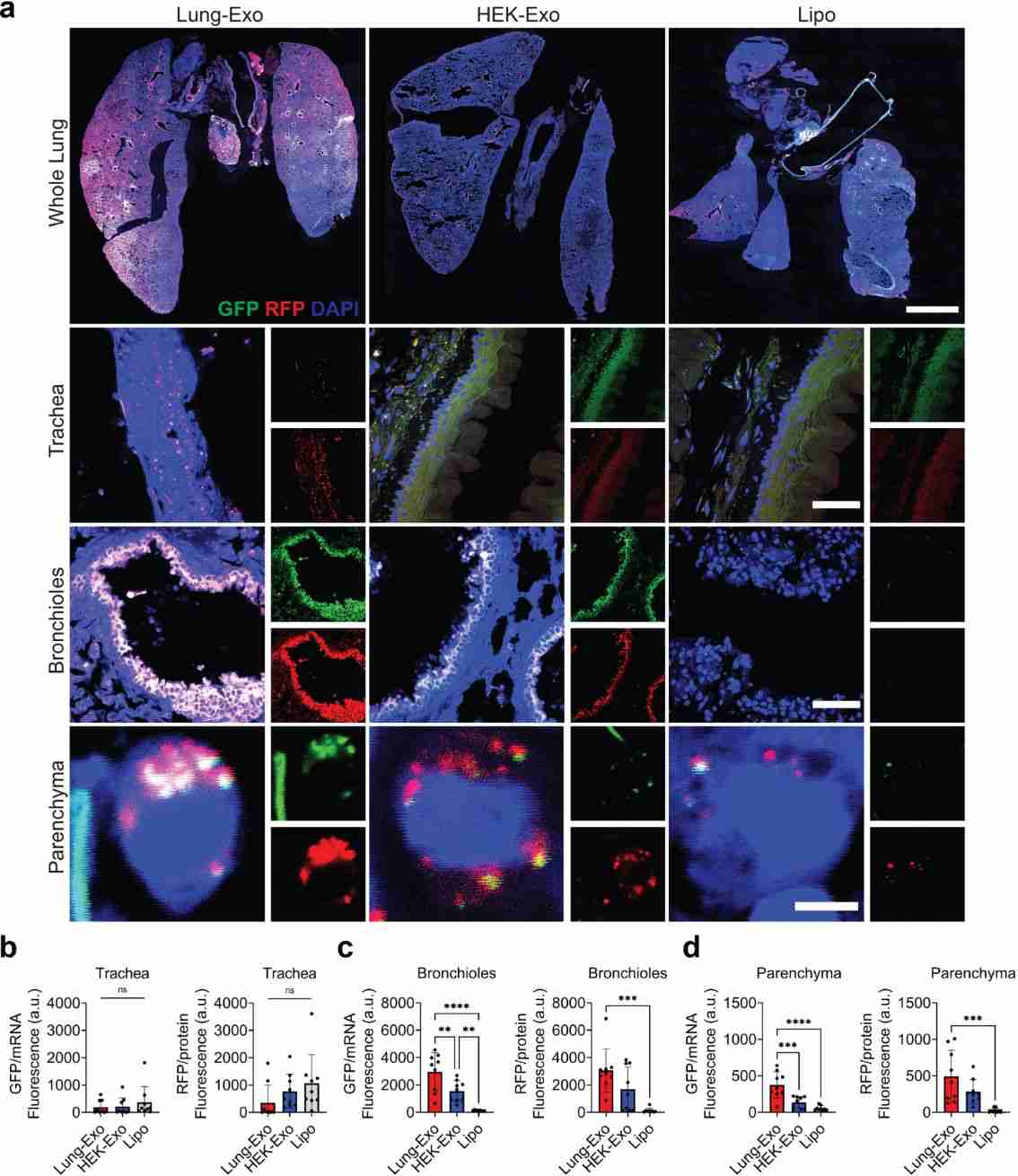 Figure 5. mRNA and protein delivery to the trachea, bronchioles, and parenchyma. (L Volpini, et al., 2023)
Figure 5. mRNA and protein delivery to the trachea, bronchioles, and parenchyma. (L Volpini, et al., 2023)
Our Products
As a novel therapy, mRNA has outstanding advantages over gene therapy or alternative therapies. Exosomes, as a novel mRNA delivery vehicle, have the potential to increase extracellular stability and make mRNA transfection more efficient. Recently, Exosome-based mRNA delivery systems have been used for the treatment of cancer and chronic diseases, including Parkinson's disease, leukemia, nerve sheath tumors, gliomas, and breast cancer.
As a leader in the field of exosome research, Creative Biostructure is always aware of the potential role of exosomes in drug delivery. We offer exosome products isolated from cancer cell lines and human disease-state body fluids to help clients advance research.
| Cat No. | Product Name | Source |
| Exo-CH06 | HQExo™ Exosome-MCF-7 | Exosome derived from human breast cancer, noninvasive cell line (MCF-7 cell line) |
| Exo-CH18 | HQExo™ Exosome-LnCAP | Exosome derived from human prostate adenocarcinoma (LnCAP cell line) |
| Exo-CH24 | HQExo™ Exosome-SK-BR-3 | Exosome derived from human breast carcinoma cell line (SK-BR-3) |
| Exo-HDBF-01 | HQExo™ Exosome-SDH-Alzheimer's plasma | Exosome derived from Single Donor Human Alzheimer's plasma |
| Exo-HDBF-03 | HQExo™ Exosome-SDH-Atopic Dermatitis plasma | Exosome derived from Single Donor Human Atopic Dermatitis plasma |
| Exo-HDBF-15 | HQExo™ Exosome-SDH-Breast Cancer Plasma exosome | Exosome derived from Single Donor Human Breast Cancer Plasma |
| Exo-HDBF-08 | HQExo™ Exosome-SDH-Crohn's Disease plasma | Exosome derived from Single Donor Human Crohn's Disease plasma |
| Explore All Exosomes Products | ||
At Creative Biostructure, we are committed to providing professional services to explore the potential functions of exosomes. If you have any questions, please feel free to contact us.
References
- Aslan C, et al. Exosomes for mRNA delivery: a novel biotherapeutic strategy with hurdles and hope. BMC Biotechnol. 2021. 21(1): 20.
- Gorshkov A, et al. Exosomes as Natural Nanocarriers for RNA-Based Therapy and Prophylaxis. Nanomaterials. 2022. 12(3): 524.
- Li Z, et al. Cell-Derived Vesicles for mRNA Delivery. Pharmaceutics. 2022. 14(12): 2699.
- You Y, et al. Intradermally delivered mRNA-encapsulating extracellular vesicles for collagen-replacement therapy. Nat Biomed Eng. 2023. 7(7): 887-900.
- Popowski KD, et al. Inhalable exosomes outperform liposomes as mRNA and protein drug carriers to the lung. Extracell Vesicle. 2022. 1: 100002.
