Flow Cytometry for Exosome Characterization
Exosomes, a subset of extracellular vesicles (EVs), are emerging mediators of intercellular communication that can reflect the physiological or pathological state of cells for disease diagnosis and pharmacological research. Due to natural cell-specific targeting properties, exosomes are often designed to target macromolecules (DNA and RNA) and drug delivery systems (DDS). Characterizing exosome structure is essential as it determines DDS properties such as cell or tissue affinity, stress response, uptake pathway, and drug release. Parameters such as number, size, morphology, membrane composition, and proteins (including receptors) must be considered when developing exosome-based DDS. In recent years, the most commonly used method for exosome characterization is flow cytometry.
What is Flow Cytometry?
Flow cytometry is a high technology based on flow cytometry, which analyzes a single column of cells or biological particles in a rapid linear flow state, one by one, multi-parameter, rapid qualitative and quantitative analysis. It can accurately quantitatively analyze and classify a large number of exosomes in a very short period. This technology can continuously analyze multiple parameters, such as size, density, surface markers, and content, of an individual exosome flowing through an optical or electronic detector, which provides great convenience for bioscience research.
Flow Cytometer
Flow cytometer is a cutting-edge scientific equipment integrating optics, electronics, fluid dynamics, cell chemistry, biology, immunology, laser and computers, etc. It is known as the "CT" of the laboratory. The flow cytometer comprises five parts: 1. Flow chamber and liquid flow driving system 2. Laser light source and beam forming system 3. Optical system 4. Signal detection, storage, display, and analysis system 5. Cell sorting system.
The three main components are as follows
- Fluid system - The system consists of sheath fluid and laminar flow, which narrows the sample core so that cells move in a single stream as they pass through the laser beam.
- Optical system - The optical system consists of a laser, lens, and collection optics involved in irradiating the sample stream passing through the laser and measuring the scattered light from each cell.
- Electronics and data acquisition system – It consists of photodetectors that convert the voltage of the laser beam and provide data to be processed and analyzed using computer software.
What is the Principle of Flow Cytometry?
Flow cytometry operates on the principle of light scattering, excitation, and emission. The fluorescently labeled components will be excited and emit fluorescence when passing through the laser beam, and at the same time generate scattered light (including forward and side scattered light) signals, which are absorbed by a photomultiplier fluorescence detector placed at an angle of 90° and a photodiode scattered light detector placed at an angle of forward direction, respectively, and converted into electrical signals, which are input into a computer through analog/digital conversion to produce a series of analytical images.
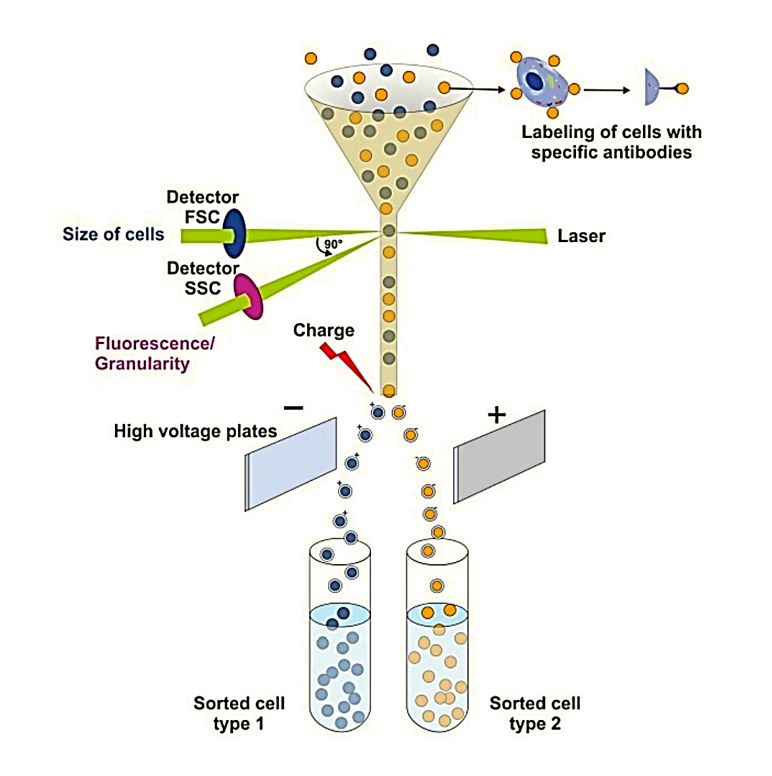 Figure 1. Principles of flow cytometry. (Drescher H, et al., 2021)
Figure 1. Principles of flow cytometry. (Drescher H, et al., 2021)
The forward scattered light parameter (FSC) reflects the size of the cell volume, the larger the cell the larger the forward; the side scattered light parameter (SSC) reflects the number of particles in the cell and the degree of complexity, the more particles, the larger the SSC.
Application of Flow Cytometry in Exosome Characterization
Embedded in the surface membrane of exosomes are biologically active ligands and enzymes that play critical roles in regulating anti-tumor immune responses. To research the surface cargo of exosomes, flow cytometry analysis has replaced the semi-quantitative western blotting widely used for exosome characterization. On-bead flow cytometry of exosomes using conventional cytometers requires carefully executed titration of bead/Abs/exosome ratios, instrument calibration, and data interpretation. In recent years, researchers have demonstrated the usefulness of flow cytometry for protein detection and quantification of exosomes isolated from cancer cell line supernatants or plasma.
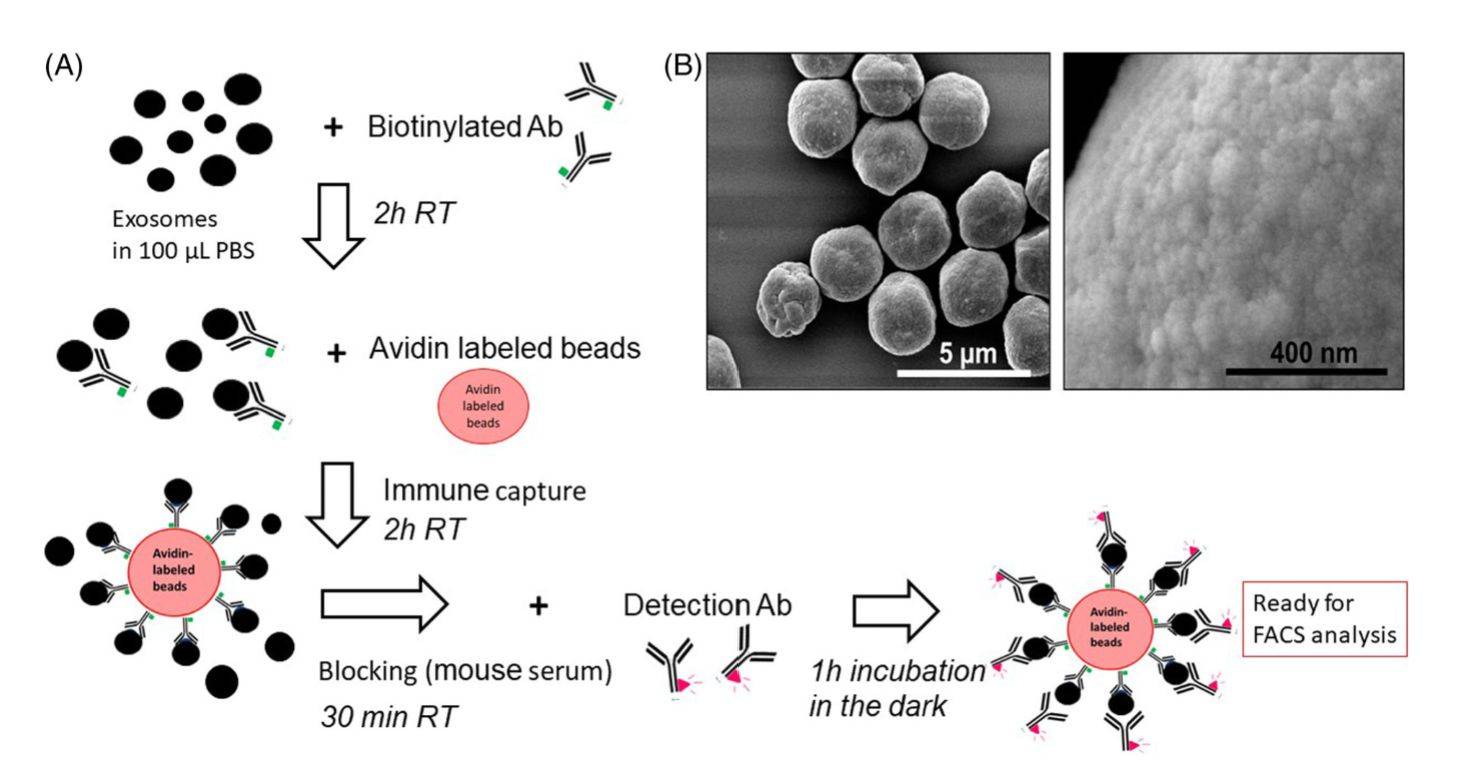 Figure 2. Immune capture and detection of the exosome cargo by on-bead flow cytometry. (Theodoraki MN, et al., 2021)
Figure 2. Immune capture and detection of the exosome cargo by on-bead flow cytometry. (Theodoraki MN, et al., 2021)
| Cat No. | Product Name | Source |
| Exo-CH15 | HQExo™ Exosome-BLCL21 | Exosome derived from EBV transformed lymphoblastoid B cells (BLCL21 cell line) |
| Exo-CH08 | HQExo™ Exosome-BPH-1 | Exosome derived from human benign prostatic hyperplasia-1 (BPH-1 cell line) |
| Exo-CH22 | HQExo™ Exosome-HCT116 | Exosome derived from human colorectal carcinoma cell line initiated from an adult male (HCT116 cell line) |
| Exo-CH17 | HQExo™ Exosome-HT29 | Exosome derived from human adenocarcinoma (HT29 cell line) |
| Exo-CH18 | HQExo™ Exosome-LnCAP | Exosome derived from human prostate adenocarcinoma (LnCAP cell line) |
| Explore All Exosomes Isolated from Cancer Cell Lines | ||
Steps for Exosome Characterization by Flow Cytometry
1. Sample preparation - Clients provide exosomes or tissue samples (we will provide exosome isolation service). The exosomes to be detected are fixed, washed, and fluorescently stained for subsequent detection and analysis.
2. Sample passes through the laser beam - The processed sample is added to the flow chamber. The exosome sample in the flow chamber passes through the laser beam at millions per second.
3. Fluorescence detection - As the sample passes through the laser beam, the laser beam excites antigens, antibodies, or other markers on the surface of the exosome to emit fluorescence, and these fluorescence signals are collected by the photomultiplier tube and converted to electrical signals.
4. Data processing and analysis - Flow cytometry can simultaneously record multiple parameters of a single exosome, including fluorescence intensity, size, and shape, allowing for multi-parameter quantitative analysis.
Advanced Technology for Flow Cytometry
- Multi-Color Flow Cytometry
This technique analyzes subpopulations of target cells based on multiple experimental parameters. In immunology, it detects specific populations of immune cells at the single-cell level by combining quantitative proteomics methods.
- Full Spectrum (Spectral) Flow Cytometry
This technique captures the entire emission spectrum of a fluorescent dye, providing more in-depth information. Has advantages such as reduced compensation requirements, increased panel size, and richer data sets.
- Mass Spectrometry or Time-of-Flight Flow Cytometry (CyTOF)
This is the next generation of flow cytometry protocols involving the use of metal-labeled antibodies for morphological and functional analysis of single cells.
Trends in Flow Cytometry
- Developing flow cytometers with higher sensitivity, higher resolution, and higher throughput.
- Explore new fluorescent dyes, laser light sources, and detection technologies.
- Develop smarter and more efficient software tools to realize automated data processing and analysis.
- Focus on multi-dimensional and multi-indicator analysis of single cells to reveal cellular heterogeneity and disease pathogenesis.
- Promote the wide application of flow cytometry in clinical diagnosis, efficacy monitoring, and individualized treatment.
Highlights of Flow Cytometry
- High throughput - Flow cytometry can detect and analyze at a rate of millions of cells per second, thus allowing rapid processing of a large number of cell samples.
- Multi-parameter quantitative analysis - Flow cytometry can record multiple parameters simultaneously, such as fluorescence intensity, cell size, and shape, thus enabling multi-parameter quantitative analysis to obtain more biological information.
- High sensitivity - Flow cytometers can detect weak fluorescence signals and therefore can detect low concentrations of antigens, antibodies, or other markers with high sensitivity.
- Highly automated - The detection and analysis process of flow cytometry is highly automated, which can reduce human error and improve the reliability and reproducibility of experimental results.
Creative Biostructure specializes in exosome characterization services based on cutting-edge flow cytometry technology. Recognizing that exosomes play a critical role in a variety of biological processes, we aim to provide our clients with efficient and accurate exosome analysis services. We help clients gain a broader and deeper understanding of the diversity and function of exosomes. Please feel free to contact us for a formal quote.
References
- Drescher H, et al. Flow Cytometry: A Blessing and a Curse. Biomedicines. 2021. 9(11): 1613.
- Theodoraki MN, et al. Evaluation of Exosome Proteins by on-Bead Flow Cytometry. Cytometry A. 2021. 99(4): 372-381.
- Maia J, et al. Employing Flow Cytometry to Extracellular Vesicles Sample Microvolume Analysis and Quality Control. Front Cell Dev Biol. 2020. 8: 593750.