Structural and Functional Analysis of Membrane Protein Complexes
Membrane proteins are fundamental to various cellular processes, including signal transduction, transport, and cell recognition. Many of these proteins do not function in isolation but form complexes that enable them to execute their roles more effectively. The study of membrane protein complexes' structure and function is thus crucial for understanding their biological significance.
Structure of Membrane Protein Complexes
Membrane Protein Organization
Membrane proteins can be organized into complexes through various interactions, including hydrophobic interactions, hydrogen bonding, and van der Waals forces. These interactions facilitate the proper orientation and stabilization of proteins within the lipid bilayer, which is critical for their function.
Types of Membrane Protein Complexes
- Homooligomeric Complexes: These complexes consist of identical subunits that come together to form functional assemblies. Examples include ion channels like the homo-oligomeric potassium channels, which form pore structures critical for ion transport.
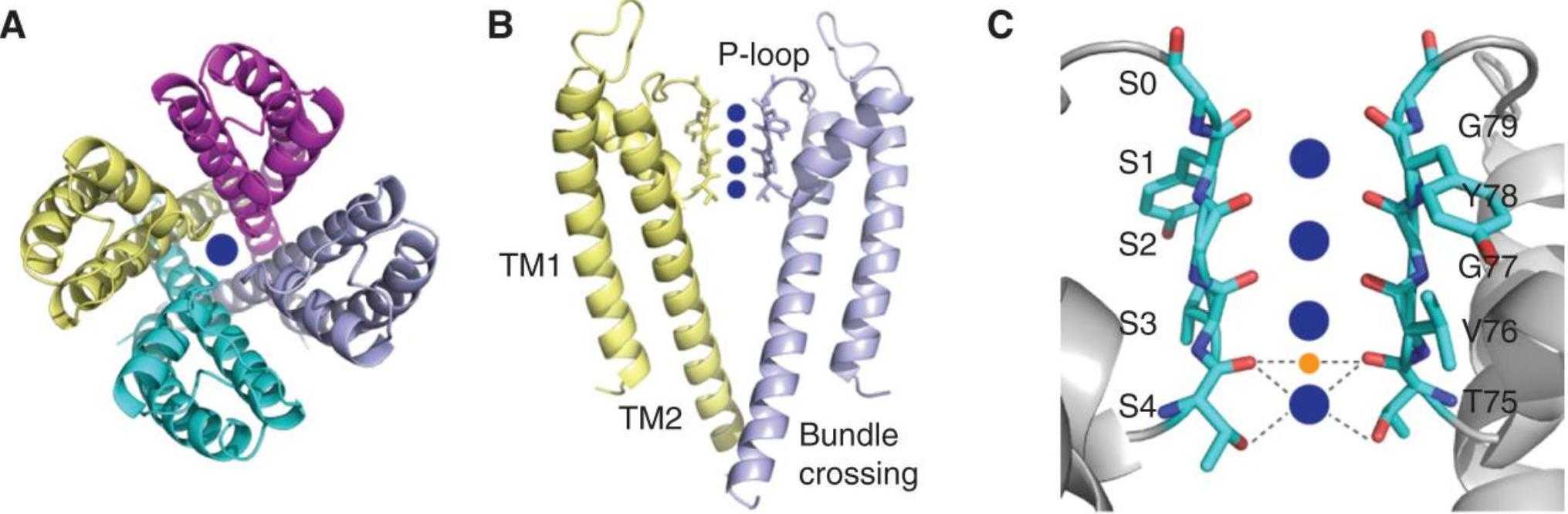 Figure 1. The structure shows that KcsA is a symmetric tetramer made of four identical polypeptide chains, with a central pore and conserved residues forming a selective filter. (Kim D M, et al., 2016)
Figure 1. The structure shows that KcsA is a symmetric tetramer made of four identical polypeptide chains, with a central pore and conserved residues forming a selective filter. (Kim D M, et al., 2016)
- Heterooligomeric Complexes: Comprising different subunits, these complexes often have more sophisticated functions. For instance, the photosystem II complex in chloroplasts is made up of multiple different protein subunits and cofactors, working together to drive the process of photosynthesis.
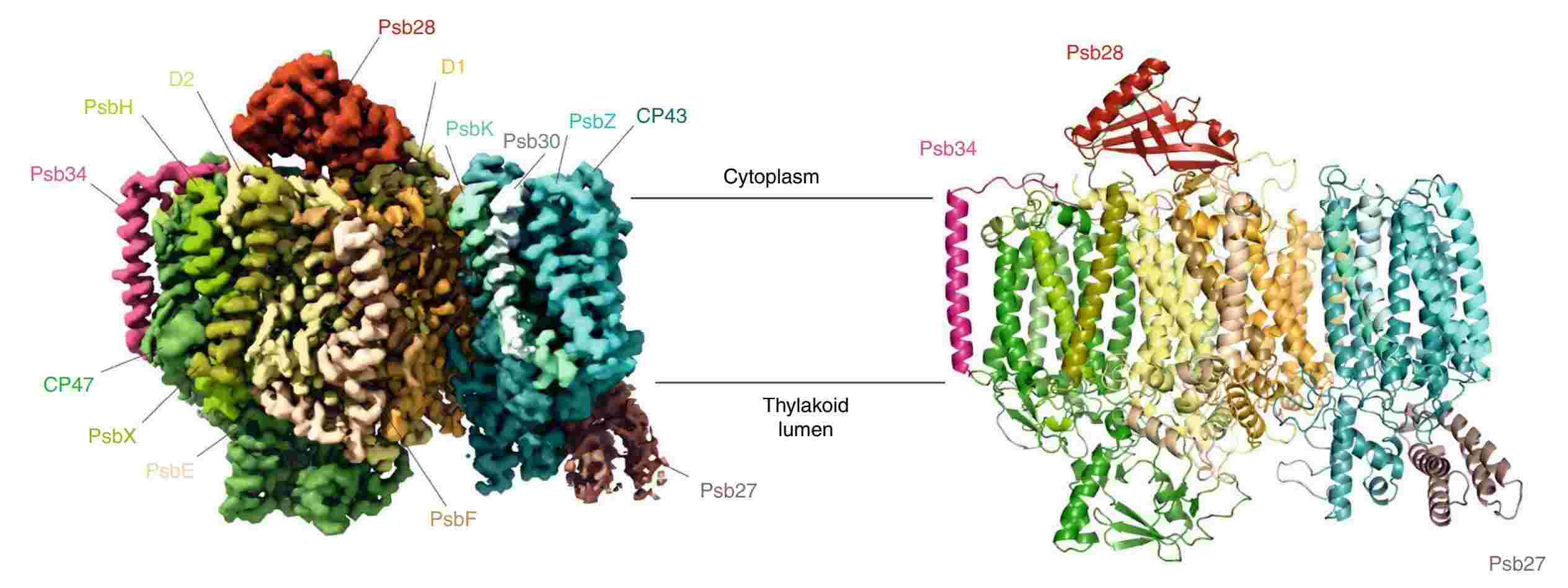 Figure 2. Cryo-EM map of a photosystem II assembly intermediate from T. elongatus. (Zabret J, et al., 2021)
Figure 2. Cryo-EM map of a photosystem II assembly intermediate from T. elongatus. (Zabret J, et al., 2021)
- Integral and Peripheral Interactions: Integral membrane proteins span the lipid bilayer, often forming complexes with peripheral proteins that attach loosely to the membrane. These associations are significant in signal transduction pathways, where peripheral proteins might shuttle between the cytosol and membrane, relaying signals.
Methods for Assembly and Characterization of Membrane Protein Complexes
| Items | Techniques | Description |
| Assembly Techniques | Co-expression Systems | The co-expression of multiple proteins in a single host cell can facilitate the natural assembly of protein complexes. This method is widely used in recombinant DNA technology, employing bacterial, yeast, or mammalian expression systems. |
| Reconstitution into Lipid Bilayers | Protein complexes can be reconstituted into artificial lipid bilayers or liposomes to examine their function in a controlled environment. This approach is beneficial for studying transport mechanisms and protein-protein interactions within a membrane context. | |
| Cell-free Systems | With cell-free synthesis, membrane protein complexes can be assembled in vitro, bypassing the complexity of cellular systems. This method allows for precise control over the protein synthesis environment and the incorporation of labeled or modified amino acids for structural studies. | |
| Characterization Methods | Cryo-Electron Microscopy (Cryo-EM) | Cryo-EM has emerged as a powerful tool for studying membrane protein complexes at nearly atomic resolution without the need for crystallization. By rapidly freezing the protein complex and imaging it with an electron microscope, researchers can visualize the structure in a state that closely resembles its natural environment. |
| Nuclear Magnetic Resonance (NMR) Spectroscopy | NMR can provide detailed information about the dynamics and structure of membrane protein complexes in a native-like environment. It is particularly useful for studying small to medium-sized proteins and complexes in solution. | |
| Fluorescence Resonance Energy Transfer (FRET) | FRET is used to study the interactions between proteins within a complex. By tagging proteins with donor and acceptor fluorophores, researchers can measure energy transfer between them, providing insights into the distances and conformational changes within the complex. | |
| Mass Spectrometry | Mass spectrometry (MS) techniques, like cross-linking MS and hydrogen-deuterium exchange MS, are valuable for elucidating the composition and topology of membrane protein complexes. These approaches can identify interaction sites and conformational changes upon complex formation. | |
| Atomic Force Microscopy (AFM) | AFM is a powerful tool for investigating the nanoscale architecture and dynamics of protein assembly. Unlike techniques that rely on electromagnetic waves, AFM uses a mechanical probe to scan the surface of a sample at nanometer resolution. This method is particularly advantageous for studying membrane protein complexes as it allows for in situ imaging under physiological conditions, capturing dynamic interactions and conformational changes. |
Functional Analysis of Membrane Protein Complexes
Ion Channels and Transporters
Membrane protein complexes such as ion channels and transporters play pivotal roles in maintaining cellular homeostasis. For example, the ATP-binding cassette (ABC) transporters, which are heterooligomeric complexes, manage the transport of various substrates across cellular membranes, influencing processes from drug resistance to lipid transport. Understanding their structure helps decipher the mechanisms of substrate binding and translocation, with implications for drug development and disease treatment.
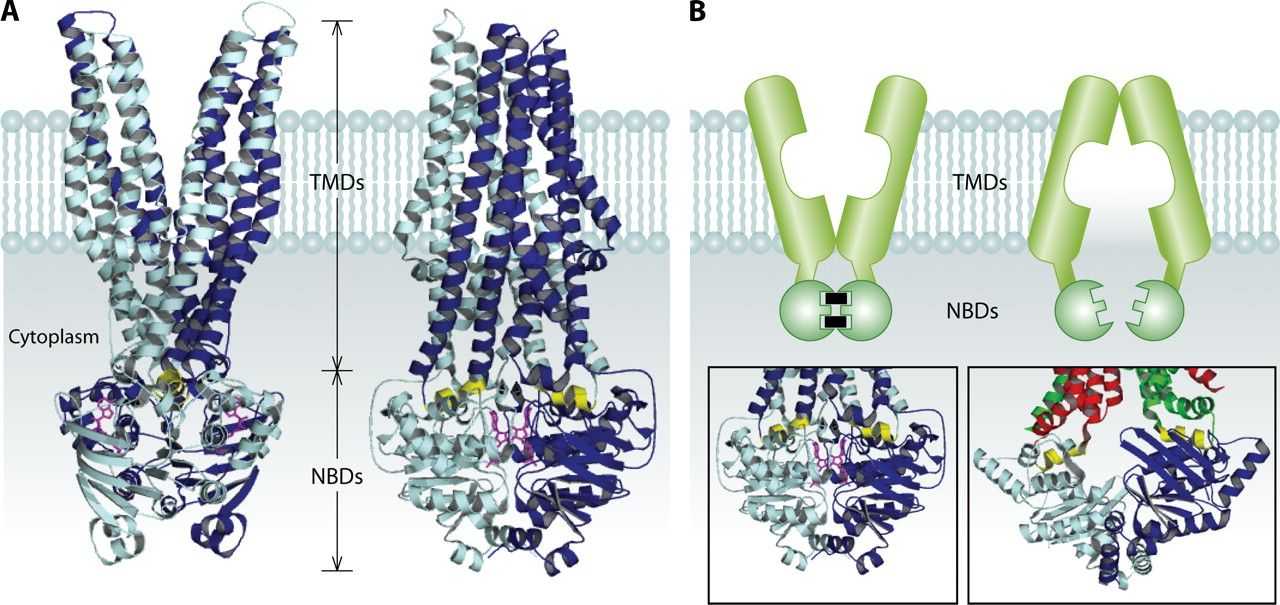 Figure 3. Model for ABC exporters. (Cuthbertson L, et al., 2010)
Figure 3. Model for ABC exporters. (Cuthbertson L, et al., 2010)
Signal Transduction
Receptor complexes on cellular membranes are key players in signal transduction pathways. G protein-coupled receptors (GPCRs), when bound to their respective ligands, undergo conformational changes that activate associated G proteins, initiating a cascade of intracellular events. Structural analysis of GPCR complexes has revealed how specific receptor conformations can selectively engage different G proteins or other partners, elucidating the mechanistic basis of diverse signaling outcomes.
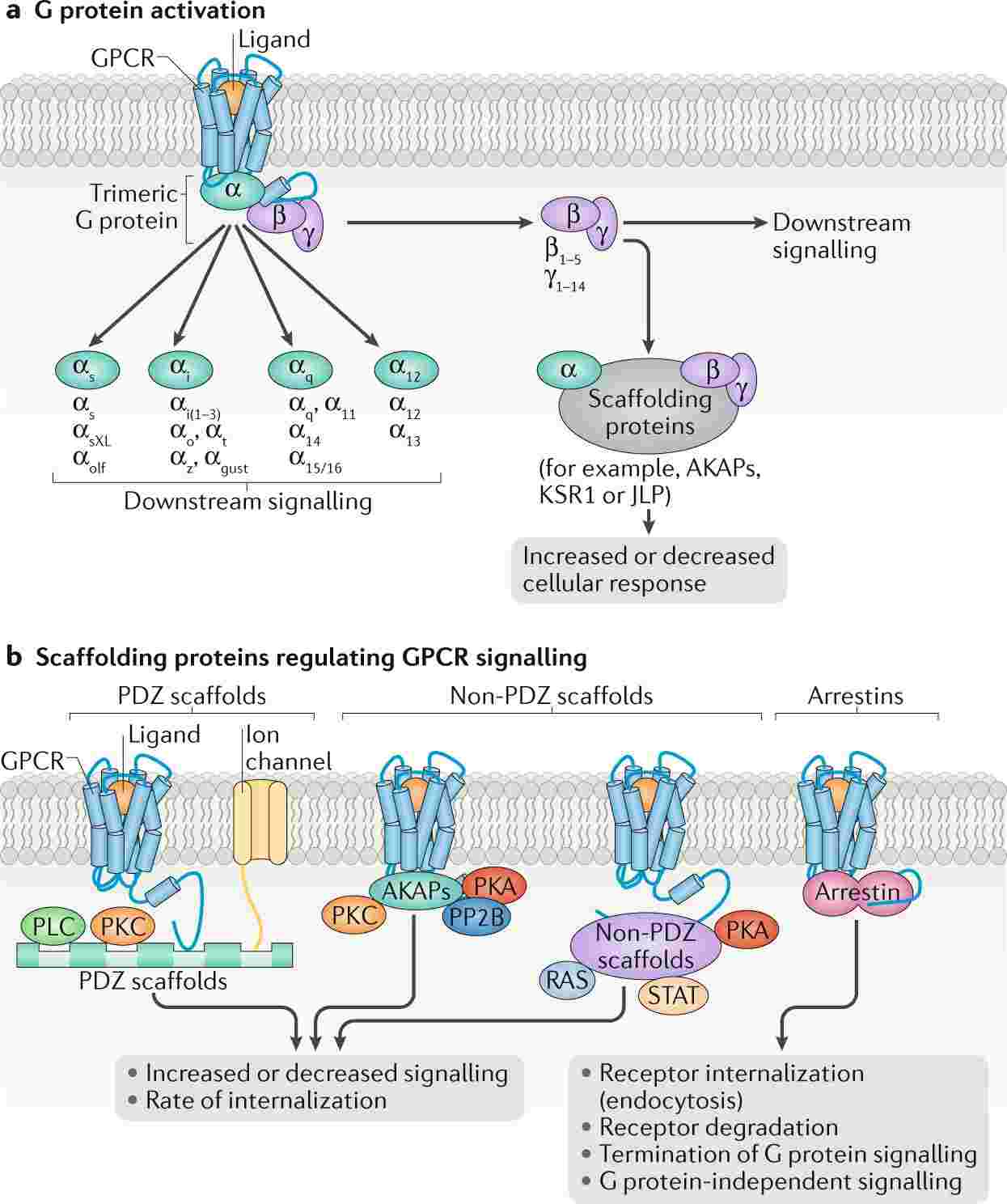 Figure 4. Schematic illustration of GPCR signalling. (Wootten D, et al., 2018)
Figure 4. Schematic illustration of GPCR signalling. (Wootten D, et al., 2018)
Photosynthesis
Photosynthetic complexes such as Photosystem I and II are paramount for converting light energy into chemical energy. These complexes are composed of multiple protein subunits and cofactors, precisely arranged to facilitate electron transfer and energy conversion. Structural studies have illuminated how light-induced excitations are captured and transferred efficiently, providing insights into optimizing artificial photosynthetic systems for renewable energy production.
Cellular Adhesion
Membrane protein complexes also mediate cell-cell and cell-matrix interactions, crucial for tissue formation and integrity. Cadherins, a family of homophilic cell adhesion molecules, form complex structures that bridge adjacent cells, influencing tissue dynamics and cellular signaling. Integrins, another family of adhesion receptors, form complexes with extracellular matrix proteins and intracellular cytoskeletal components, playing essential roles in cellular movement and signal transduction.
Biological Significance of Membrane Protein Complexes
Cellular Communication and Coordination
The formation of protein complexes allows for efficient communication and coordination within and between cells. Membrane protein complexes ensure that signals are transmitted rapidly and accurately, which is essential for processes such as immune responses, neural communication, and hormonal regulation.
Functional Versatility
By forming complexes, membrane proteins can achieve a level of functional versatility that individual proteins cannot. Complex formation can modulate the activity, specificity, and regulatory properties of the component proteins, enabling them to adapt to various physiological conditions and requirements.
Regulation of Protein Activity
Protein complex formation often serves as a regulatory mechanism. For instance, the binding of a regulatory subunit can change the conformation and activity of an enzyme, acting as a switch to turn metabolic pathways on or off. This regulation is critical for maintaining cellular homeostasis and responding to environmental changes.
Creative Biostructure offers cutting-edge techniques and methodologies for the study of membrane protein complexes. Our advanced services include Cryo-EM, NMR spectroscopy, FRET, mass spectrometry (MS), and AFM. These state-of-the-art technologies enable detailed structural and functional analysis, facilitating insights into protein assembly, dynamics, and interactions crucial for understanding cellular processes and developing therapeutic strategies. If you are interested in our solutions, please feel free to contact us for more information.
References
- Cuthbertson L, Kos V, Whitfield C. ABC transporters involved in export of cell surface glycoconjugates. Microbiology and Molecular Biology Reviews. 2010. 74(3): 341-362.
- Kim D M, Nimigean C M. Voltage-gated potassium channels: a structural examination of selectivity and gating. Cold Spring Harbor Perspectives in Biology. 2016. 8(5): a029231.
- Wootten D, Christopoulos A, Marti-Solano M, et al. Mechanisms of signalling and biased agonism in G protein-coupled receptor. Nature Reviews Molecular Cell Biology. 2018. 19(10): 638-653.
- Zabret J, Bohn S, Schuller S K, et al. Structural insights into photosystem II assembly. Nature Plants. 2021. 7(4): 524-538.