Structural Research of Rhodaneses
Rhodaneses (Rhd) are mitochondrial enzymes that can detoxify by converting cyanide (CN-) into thiocyanate (SCN-). This reaction is essential for the treatment of cyanide exposure. Therefore, the clinical use of thiosulfate solutions as an antidote to cyanide poisoning is based on the activation of this enzyme cycle. Research has revealed that proteins containing the Rhd domain are involved in various physiological processes including metabolic pathways, signal transduction, and post-translational modification. Analyzing the structure of Rhd is an essential step in understanding its importance in physiology.
Structural Analysis of Rhd
Three-dimensional X-ray data with a resolution of 3.9 Å are collected from crystals of natural proteins, mercury-containing, and platinum-containing heavy-atom derivatives. The phases of the protein reflections are determined by isomorphic substitution. Its central parallel β-sheet layer surrounded by four to five α-helices can be seen in the structure. Two highly conserved structural motifs are identified within the Rhd domain, located at the end of the β1 strand, corresponding to the DxR motif of unknown function, and at the end of the β3 strand, corresponding to the (C/D) x 4/5 (R/T) loop motif. Proteins containing Rhd domains also exhibit variability, with one or more Rhd domains fused or not to other functional domains.
Unique class I Rhd enzyme to date - TSTD1
TSTD1 consists of four exons and three introns and generates three transcripts by selective splicing. In vitro, research has shown that the TSTD1-1 protein has an affinity for KCN, GSH, Cys, or homocysteine (HCys) in the millimetre range. Furthermore, potential mechanistic crosstalk between TSTD1 and proteins with persulfide dioxygenase (PDO) activity suggests that TSTD1 proteins are part of the mitochondrial sulfide oxidizing unit complex (SOU complex), a major player in mammalian cellular detoxification. The high-resolution crystal structure of the TSTD1-1 isoform reveals a typical Rhd fold consisting of five chain-like β-folds surrounded by six α-helices.
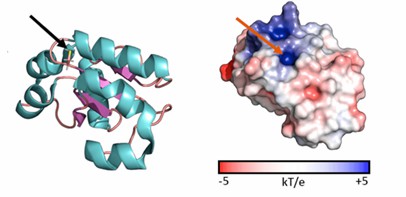 Figure 1. Cartoon and surface representations of the TSTD1 structure. (Alsohaibani R, et al., 2023)
Figure 1. Cartoon and surface representations of the TSTD1 structure. (Alsohaibani R, et al., 2023)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Sulfur-substituted rhodanese (orthorhombic form) | Bos taurus | X-ray diffraction | 2.3 Å | 1BOH |
| N-terminally truncated rhodanese | Bos taurus | X-ray diffraction | 2.2 Å | 1BOI |
| The complex between rhodanese and lipoate | Bos taurus | X-ray diffraction | 2.01 Å | 1DP2 |
| Rhodanese | Thermus thermophilus | X-ray diffraction | 1.7 Å | 1UAR |
| Sulfurtransferase | Azotobacter vinelandii | X-ray diffraction | 1.8 Å | 1E0C |
| Sulfurtransferase in complex with hypophosphite | Azotobacter vinelandii | X-ray diffraction | 2.05 Å | 1H4K |
| Sulfurtransferase in complex with phosphate | Azotobacter vinelandii | X-ray diffraction | 2.1 Å | 1H4M |
| Active site structural features for chemically modified forms of rhodanese | Bos taurus | X-ray diffraction | 2 Å | 1ORB |
| Bovine liver rhodanese | Bos taurus | X-ray diffraction | 2.5 Å | 1RHD |
| Sulfur-substituted rhodanese | Bos taurus | X-ray diffraction | 1.36 Å | 1RHS |
| Rhodanese (thiosulfate: cyanide sulfurtransferase) | Bos taurus | X-ray diffraction | 1.99 Å | 2ORA |
| Thiosulfate sulfurtransferase amino acids 2-297 | Homo sapiens | X-ray diffraction | 3.4 Å | 8AGF |
| Thiosulfate sulfurtransferase amino acid 1-291 in high resolution | Homo sapiens | X-ray diffraction | 1.279 Å | 8BGQ |
| Thiosulfate sulfurtransferase | Bos taurus | X-ray diffraction | 1.5 Å | 8Q5Z |
| Thiosulfate sulfurtransferase | Pseudomonas aeruginosa | X-ray diffraction | 1.9 Å | 1YT8 |
| Rhodanese-like domain | Staphylococcus aureus subsp. aureus COL | X-ray diffraction | 2 Å | 3IWH |
| GlpE sulfurtransferase | Escherichia coli BL21(DE3) | X-ray diffraction | 1.1 Å | 1GMX |
| Single domain sulfurtransferase RDL2 | Saccharomyces cerevisiae S288C | X-ray diffraction | 2.471 Å | 6K6R |
| Rhodanese domain of YgaP treated with SNOC | Escherichia coli | X-ray diffraction | 1.5 Å | 5HBP |
| Rhodanese | Escherichia coli | SOLUTION NMR | / | 2JTQ |
| Rhodanese persulfide | Escherichia coli | SOLUTION NMR | / | 2JTR |
| Rhodanese with anions | Escherichia coli | SOLUTION NMR | / | 2JTS |
| The cytoplasmic rhodanese domain of the inner membrane protein YgaP | Escherichia coli K-12 | SOLUTION NMR | / | 2MOI |
| Polysulfide-sulfur Transferase Homodimer | Wolinella succinogenes | SOLUTION NMR | / | 1QXN |
Table 1. Structural research of rhodaneses.
Creative Biostructure, as a leading enterprise in analyzing biological molecular structures, provides clients with a complete set of cutting-edge protein structure analysis services. Our X-ray crystallography and NMR spectroscopy technologies can determine the high-resolution structure of the rhodaneses.
Our team of scientists has unparalleled rich experience and a commitment to excellence, and they are passionately committed to providing high-quality results. We utilize the most advanced equipment and technology to ensure that our results are both accurate and highly reliable. We are always ready to provide clients with valuable insights into the complex structure and function of rhodaneses. Please contact us for more details.
References
- Alsohaibani R, et al. Rhodanese-Fold Containing Proteins in Humans: Not Just Key Players in Sulfur Trafficking. Antioxidants (Basel). 2023.12(4):843.
- Bordo D, Bork P. The rhodanese/Cdc25 phosphatase superfamily. Sequence-structure-function relations. EMBO Rep. 2002.3(8):741-746.
- Liu D, et al. Structure of TBC1D23 N-terminus reveals a novel role for rhodanese domain. PLoS Biol. 2020.18(5): e3000746.