Drug delivery systems (DDS) are engineered technologies designed to transport therapeutic agents to specific sites within the body, optimizing efficacy while minimizing side effects. The development of advanced DDSs has revolutionized modern medicine by improving drug bioavailability, controlled release, and targeted therapy.
Creative Biostructure provides an in-depth exploration of drug delivery methods and systems, their underlying mechanisms, comparisons of different techniques, recent research advances, and future challenges. One of the most promising ways is exosomes as drug carriers, we offer exosome service and high-quality exosome products.
Drug Delivery Routes
Oral Drug Delivery System
Oral drug delivery remains the most preferred method due to ease of administration and patient compliance. Common dosage forms include tablets, capsules and liquid formulations. However, challenges such as first-pass metabolism and poor solubility limit bioavailability. Innovative approaches such as nanoparticles, liposomes and prodrugs have been introduced to improve absorption and efficacy.
Parenteral Drug Delivery
Parenteral drug delivery bypasses the digestive system, providing rapid onset of action and precise dosage control. Intravenous (IV) administration provides immediate systemic circulation, while intramuscular (IM) and subcutaneous (SC) injections provide sustained release. Biodegradable polymers and microsphere-based carriers have improved the sustained delivery of biologics and vaccines.
Transdermal Drug Delivery Systems
Transdermal drug delivery (TDD) enables the absorption of drugs through the skin, offering non-invasive and controlled release options. Patch-based systems, microneedles, and lipid-based formulations facilitate drug penetration while reducing systemic toxicity. Recent advances include iontophoresis and sonophoresis techniques to enhance permeability.
Pulmonary Drug Delivery
Pulmonary delivery targets the respiratory system for the treatment of asthma, chronic obstructive pulmonary disease (COPD), and other lung diseases. Aerosolized drugs ensure localized action and rapid absorption into the systemic circulation. Advanced inhaler technologies improve particle size distribution for optimized deposition in the lungs.
Ocular Drug Delivery Systems
Ocular drug delivery faces barriers such as tear turnover and corneal impermeability. Innovative strategies, including nanoparticle carriers, hydrogels, and in situ gelling systems, extend drug retention and improve therapeutic outcomes for eye diseases such as glaucoma and macular degeneration.
Targeted and Controlled Drug Delivery
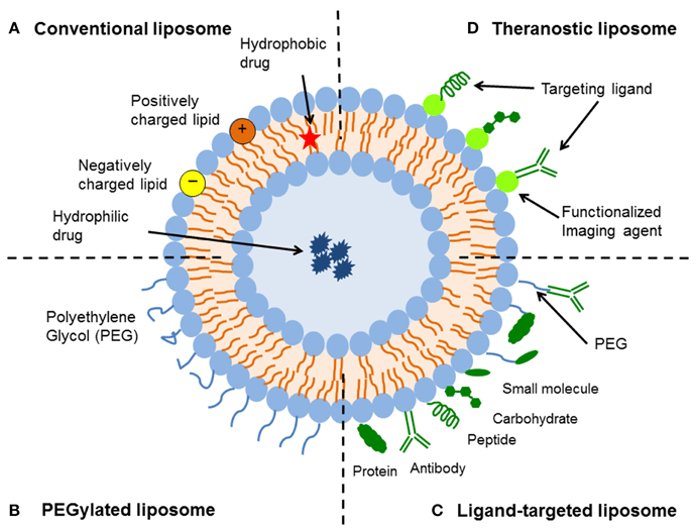
Targeted DDS uses ligands, antibodies, and stimuli-responsive materials to direct drugs to specific tissues while minimizing off-target effects. Liposomal formulations, polymeric micelles, and dendrimers have demonstrated significant improvements in site-directed therapy, particularly in oncology and gene therapy applications.
Comparison of Different Drug Delivery Routes
Each drug delivery method presents unique advantages and limitations. While oral administration ensures convenience, it may suffer from poor solubility and degradation. Parenteral delivery offers rapid systemic effects but requires skilled administration. Transdermal patches provide prolonged release but are limited to lipophilic drugs. Pulmonary systems deliver localized treatment efficiently but face challenges in dose precision. Ocular and targeted delivery systems offer precision but often require complex formulations. The selection of the appropriate DDS depends on the therapeutic objective, patient compliance and cost considerations.
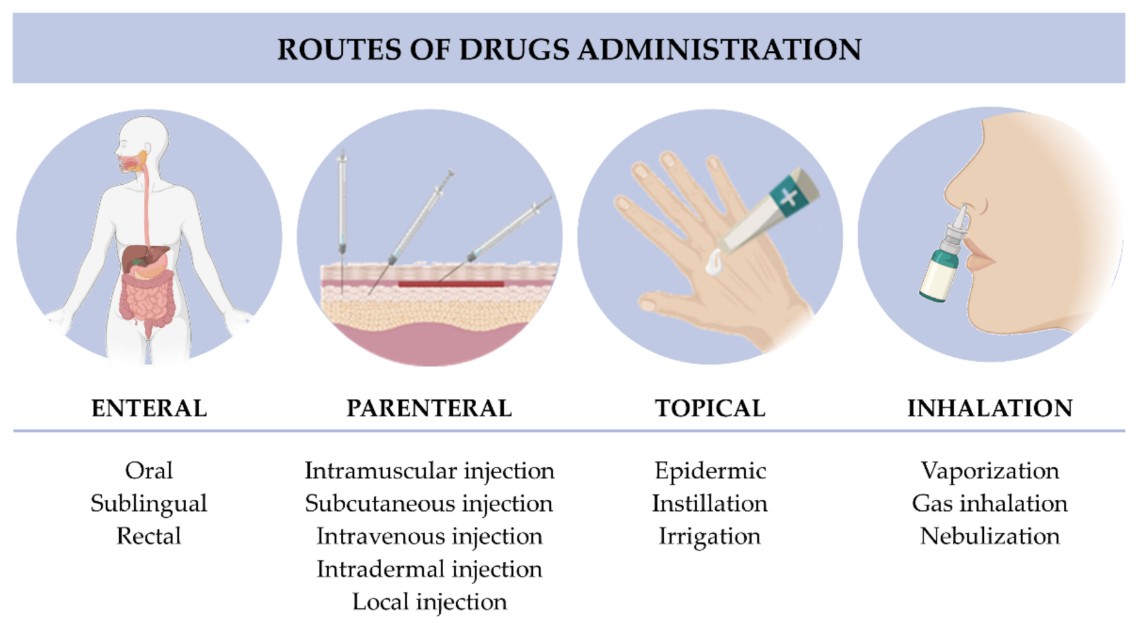 Figure. 1: Classification of routes of drugs administration. (Nunes et al., 2022)
Figure. 1: Classification of routes of drugs administration. (Nunes et al., 2022)
Targeted Drug Delivery and the Delivery of Biologic Drugs
The field of drug delivery has evolved to overcome limitations associated with conventional delivery methods, such as poor bioavailability, off-target effects, and systemic toxicity. Targeted drug delivery aims to increase the precision of therapeutics by directing drugs to specific tissues or cells. The advent of biologics - large, complex molecules derived from living organisms - has further necessitated the development of specialized delivery mechanisms. These biologics, including monoclonal antibodies, gene therapies, and RNA-based treatments, require innovative approaches to maintain stability, optimize bioavailability, and enhance therapeutic efficacy.
Mechanisms of Targeted Drug Delivery
- Passive Targeting: Passive targeting is based on the enhanced permeability and retention (EPR) effect, a phenomenon observed in tumors and inflamed tissues where leaky vasculature allows nanoparticles and macromolecular drugs to selectively accumulate. Liposomes, polymeric nanoparticles and micelles exploit this effect for the delivery of chemotherapeutics and biologics.
- Active Targeting: Active targeting involves the use of ligands, antibodies, or aptamers that recognize and bind to specific receptors on target cells. Examples include:
- Monoclonal antibodies for cancer therapy (e.g., trastuzumab targeting HER2 in breast cancer)
- Folate-conjugated nanoparticles for folate receptor-overexpressing tumors
- Peptide-functionalized carriers for site-specific drug delivery
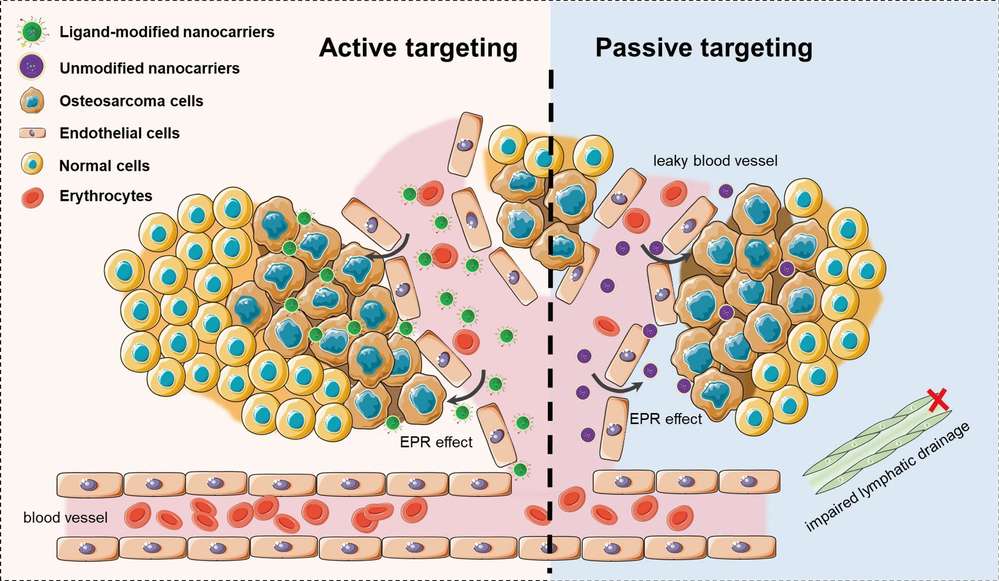 Figure. 2: A schematic illustration of active targeting and passive targeting of nano-delivery system in anti-tumor therapy. Passive targeting is achieved via enhanced permeability and retention (EPR) effects. Nanocarriers circulate in the bloodstream, extravasate, and accumulate into tumor tissue through the leaky tumor vasculature. Targeting ligand-modified nanocarriers are able to bind to highly expressed receptors on the tumor cell, exerting local drug delivery or internalization through receptor-mediated endocytosis (Shi et al., 2023)
Figure. 2: A schematic illustration of active targeting and passive targeting of nano-delivery system in anti-tumor therapy. Passive targeting is achieved via enhanced permeability and retention (EPR) effects. Nanocarriers circulate in the bloodstream, extravasate, and accumulate into tumor tissue through the leaky tumor vasculature. Targeting ligand-modified nanocarriers are able to bind to highly expressed receptors on the tumor cell, exerting local drug delivery or internalization through receptor-mediated endocytosis (Shi et al., 2023)
- Stimuli-Responsive Targeting: Stimuli-responsive systems release drugs upon exposure to specific internal (pH, enzymes, redox potential) or external (magnetic fields, ultrasound, light) stimuli. Examples include:
- pH-sensitive liposomes that release their payload in the acidic tumor environment
- Magnetic nanoparticles targeted to tumors using an external magnetic field
- Photothermal-triggered drug release from gold nanoparticles
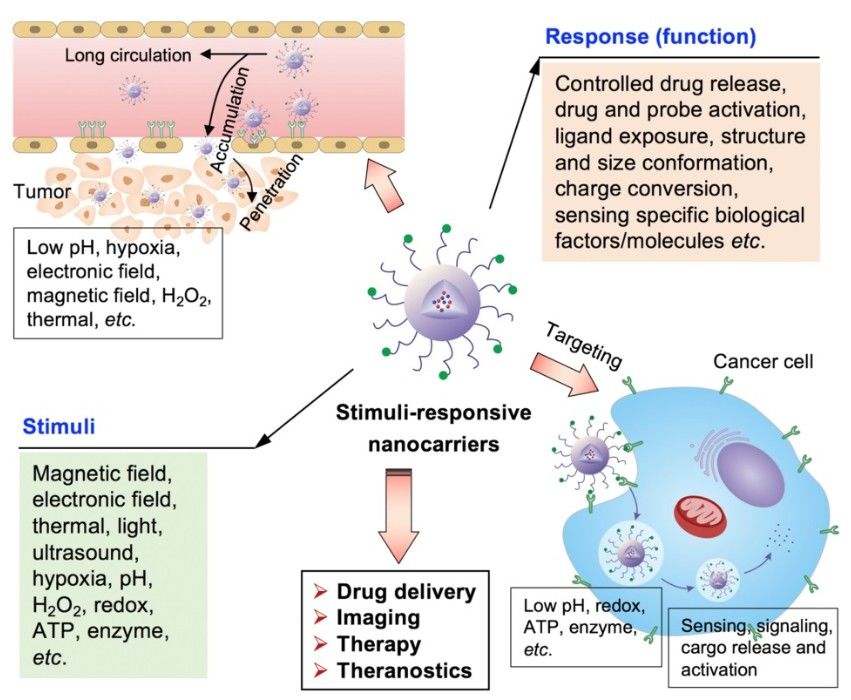 Figure. 3: The stimuli-responsive nanocarriers for drug delivery to tumors towards precision imaging, effective therapy and theragnostic. The nanocarriers could accumulate and penetrate tumors, and target cancer cells for achieving different applications and functions by responding to the external and internal stimuli. (Mi, 2020)
Figure. 3: The stimuli-responsive nanocarriers for drug delivery to tumors towards precision imaging, effective therapy and theragnostic. The nanocarriers could accumulate and penetrate tumors, and target cancer cells for achieving different applications and functions by responding to the external and internal stimuli. (Mi, 2020)
Nanotechnology-Based Delivery Systems
Nanotechnology has revolutionized drug delivery by improving drug stability, targeting precision and controlled release. Nanocarriers help protect biological drugs from degradation, improve bioavailability, and enable site-specific delivery. The most commonly used nanocarriers include:
Liposomes
These are phospholipid bilayer vesicles that encapsulate both hydrophilic and hydrophobic drugs. They are widely used for the delivery of nucleic acid-based therapies and small molecule drugs. Liposomes have been instrumental in the success of mRNA vaccines, such as the Pfizer-BioNTech and Moderna COVID-19 vaccines, where they protect mRNA from enzymatic degradation and facilitate efficient cellular uptake. Liposomal formulations are also used in chemotherapy.
Select Service & Products
Related Reading
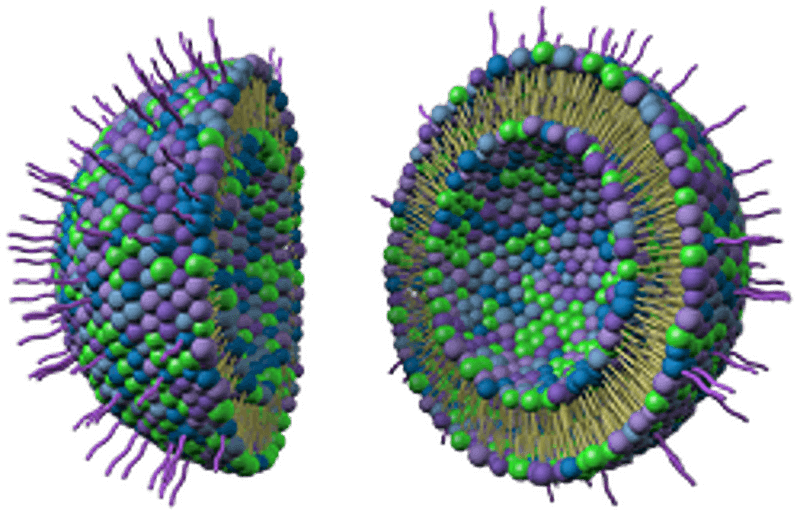
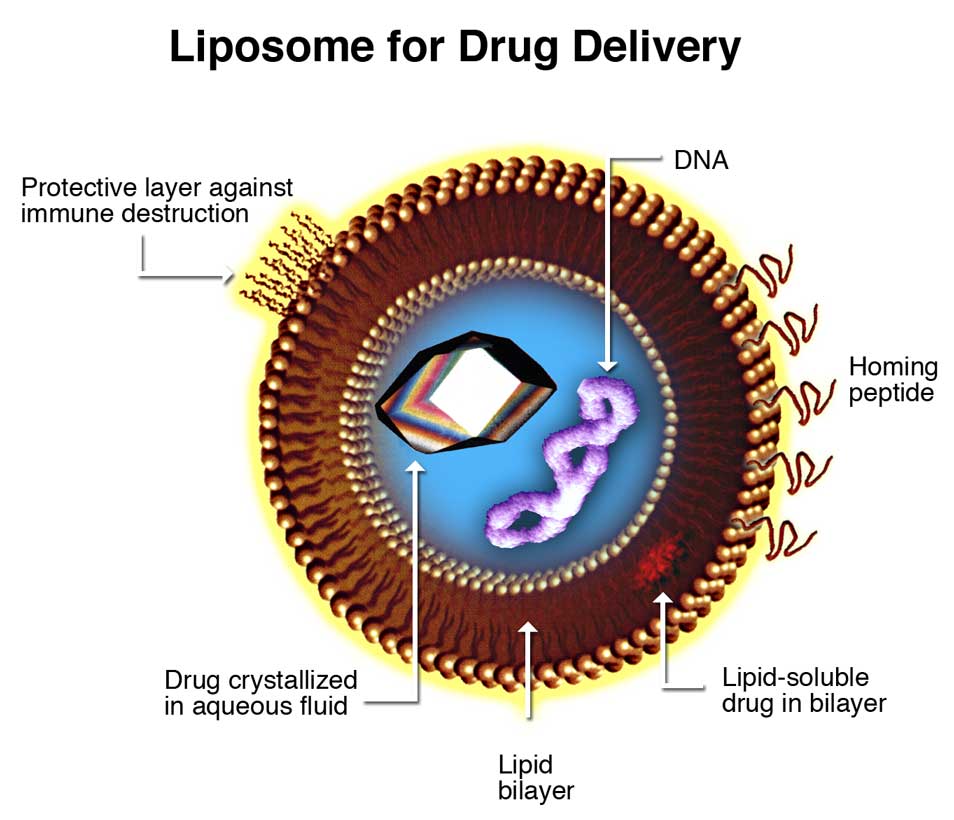 Figure. 4: Liposomes are composite structures made of phospholipids and may contain small amounts of other molecules. Though liposomes can vary in size from low micrometer range to tens of micrometers, unilamellar liposomes, as pictured here, are typically in the lower size range with various targeting ligands attached to their surface allowing for their surface-attachment and accumulation in pathological areas for treatment of disease.
Figure. 4: Liposomes are composite structures made of phospholipids and may contain small amounts of other molecules. Though liposomes can vary in size from low micrometer range to tens of micrometers, unilamellar liposomes, as pictured here, are typically in the lower size range with various targeting ligands attached to their surface allowing for their surface-attachment and accumulation in pathological areas for treatment of disease.
Nanoparticles
These include biodegradable polymers such as poly (lactic-co-glycolic acid) (PLGA), which allow for controlled drug release and prolonged circulation time. PNPs are commonly used for sustained protein delivery, peptide-based therapeutics, and gene therapy applications. The controlled release provided by PLGA nanoparticles extends the therapeutic window while minimizing systemic toxicity.
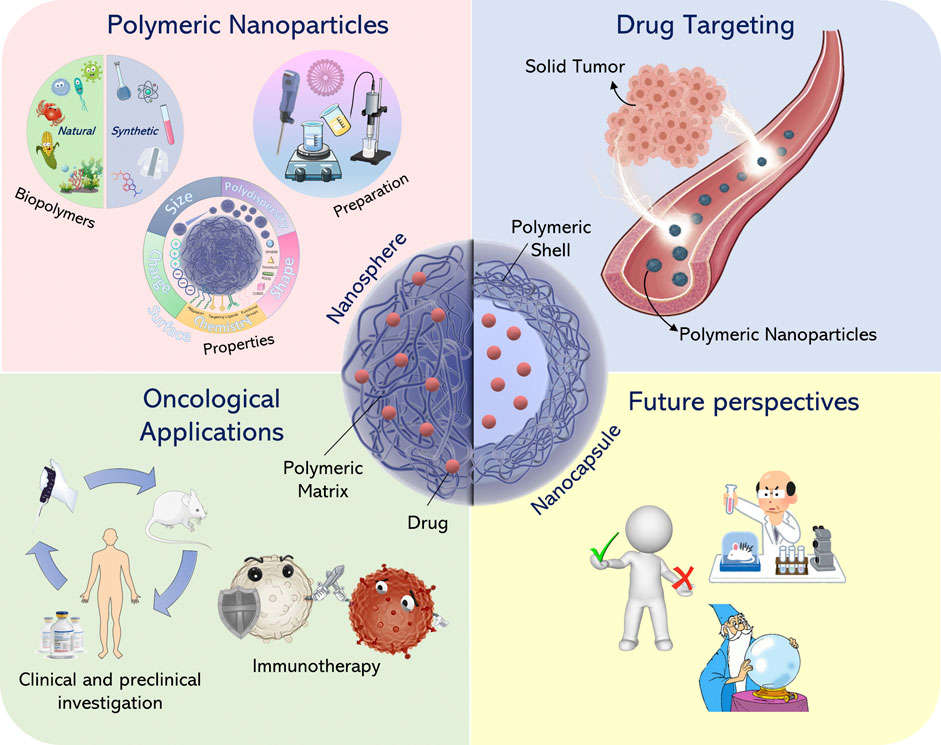 Figure. 5: Overview of the main features of polymeric nanoparticles. (Gagliardi et al., 2021)
Figure. 5: Overview of the main features of polymeric nanoparticles. (Gagliardi et al., 2021)
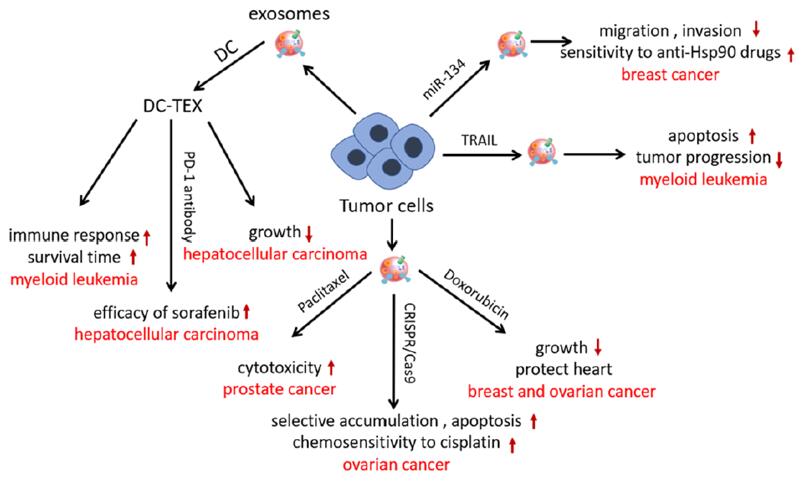
Exosomes
These naturally occurring extracellular vesicles play a critical role in intercellular communication and have been investigated as carriers for RNA- and protein-based therapeutics. Exosomes are cell-derived and have a low immunogenic profile, making them ideal for targeted drug delivery. Their use for delivery of siRNA, mRNA, and even CRISPR components for gene editing applications is being explored. Their ability to cross biological barriers, such as the blood-brain barrier, makes them promising candidates for neurodegenerative and central nervous system (CNS) disorders.
Select Service & Products
Related Reading
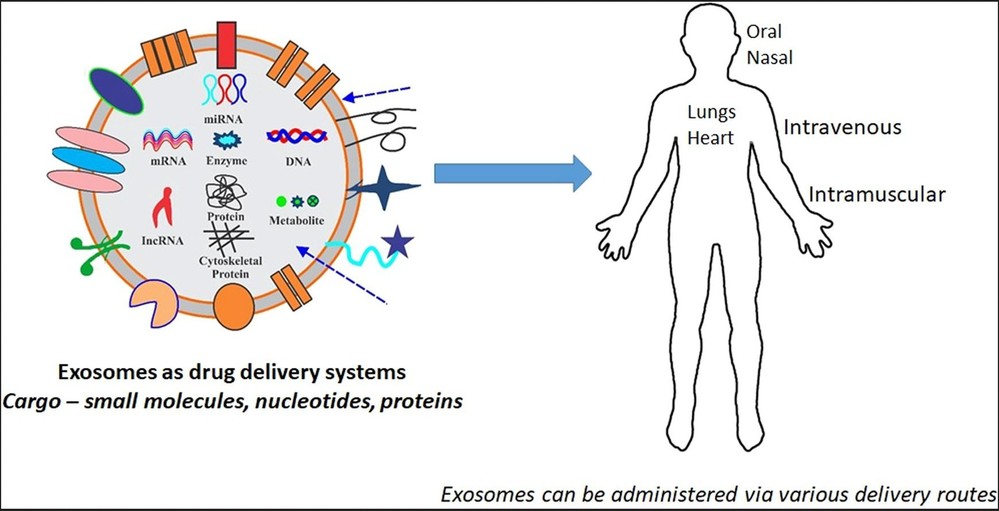 Figure. 6: Exosomes as drug delivery systems. (Patil et al., 2020)
Figure. 6: Exosomes as drug delivery systems. (Patil et al., 2020)
Lipid and Polymer-Based Carriers
Lipid- and polymer-based carriers provide stability, biocompatibility and efficient intracellular delivery of biologic drugs. These systems are particularly important for nucleic acid-based therapies, which are highly susceptible to enzymatic degradation and poor cellular uptake.
- Lipid Nanoparticles (LNPs): These advanced drug carriers have gained prominence due to their ability to encapsulate and deliver nucleic acids, proteins and hydrophobic drugs. LNPs are composed of ionizable lipids, phospholipids, cholesterol, and PEGylated lipids that help protect and efficiently transport therapeutic payloads.
- siRNA and mRNA Therapies: LNPs have played a critical role in the success of RNA-based therapies. They protect siRNA and mRNA from degradation, facilitate endosomal escape, and enhance cellular uptake. LNPs have been widely used in mRNA vaccines, such as the COVID-19 vaccine, and in siRNA therapies for genetic disorders.
- CRISPR/Cas9 Delivery: Effective gene editing requires safe and efficient delivery systems. LNPs and polymeric vesicles have shown promise in delivering CRISPR/Cas9 components to target cells, enabling precise gene modification. LNP-based delivery minimizes immune response, enhances cellular uptake, and ensures stable delivery of gene editing tools, making it a preferred method for in vivo genome editing applications.
Recent Drug Delivery Systems for Different Therapeutic Purposes
Red Blood Cell Membrane-Camouflaged Nanoparticles
This system utilizes the natural biocompatibility and long circulation time of red blood cells to enhance drug delivery. By coating nanoparticles with red blood cell membranes, immune system evasion and prolonged systemic circulation are achieved, making them effective for targeted cancer therapy and immune modulation.
Hyaluronic Acid-Based Drug Nanocarriers
Hyaluronic acid (HA) is widely used in nanocarriers due to its biocompatibility and ability to target CD44 receptors, which are overexpressed in certain cancer cells. HA-based systems enhance drug solubility, stability, and targeted delivery, making them promising for anti-inflammatory and anticancer therapies.
Hexagonal Boron Nitride Nanosheet
Hexagonal boron nitride (hBN) nanosheets exhibit high thermal and chemical stability, allowing them to function as drug carriers for sustained release and targeted therapy. Their unique structure provides a large surface area for drug adsorption, making them suitable for chemotherapy and antimicrobial applications.
Polymer-Lipid Hybrid Nanoparticles
Polymer-lipid hybrid nanoparticles (PLHNs) combine the stability of polymeric nanoparticles with the biocompatibility of liposomes. These hybrid carriers enhance drug encapsulation efficiency, controlled release, and target specificity, making them useful for cancer therapy, gene delivery, and vaccine formulations.
Self-Microemulsifying
Self-microemulsifying drug delivery systems (SMEDDS) use a mixture of oils, surfactants and co-solvents to improve the solubility and bioavailability of poorly water-soluble drugs. They are widely used in oral drug delivery to enhance gastrointestinal absorption.
In Situ Gel
In situ gel systems transform from a liquid to a gel upon administration, providing sustained drug release and localized treatment. These systems are commonly used in ophthalmic, nasal, and injectable drug formulations, improving patient compliance and therapeutic efficacy.
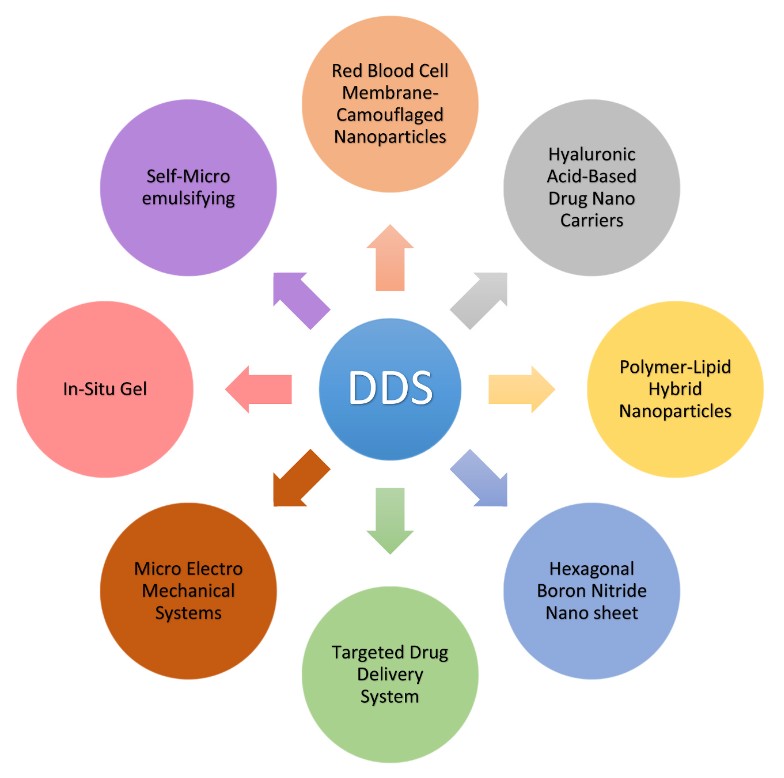 Figure. 7: Several types of recent drug delivery systems for different therapeutic purposes. (Ezike et al., 2023)
Figure. 7: Several types of recent drug delivery systems for different therapeutic purposes. (Ezike et al., 2023)
Comparison of Different Targeted Delivery Systems
| Delivery System | Mechanism | Advantages | Limitations |
|---|---|---|---|
| Liposomes | Passive and active targeting | Biocompatible, versatile | Short half-life, potential clearance by macrophages |
| Polymeric Nanoparticles | Controlled release | Enhanced stability | Potential toxicity, complex synthesis |
| Exosomes | Natural vesicles for RNA/protein delivery | Low immunogenicity, efficient cellular uptake | Limited production scalability |
| Antibody-Drug Conjugates | Targeted therapy | High specificity | Complex manufacturing, potential off-target toxicity |
| Micelles | Encapsulation of hydrophobic drugs | Improved solubility | Stability concerns |
Challenges and Future Directions
Challenges of Targeted Drug Delivery Systems
| Biological Barriers and Drug Stability | The human body has numerous physiological barriers, such as the blood-brain barrier (BBB), mucosal barriers, and immune clearance mechanisms, which can prevent the efficient delivery of drugs to target tissues. |
| Biologic drugs (e.g., proteins, peptides, and nucleic acids) are susceptible to enzymatic degradation in the bloodstream, resulting in reduced therapeutic efficacy. | |
| Nanocarriers are often subject to clearance by the mononuclear phagocyte system (MNPS), limiting their circulation time and drug bioavailability. | |
| Targeting Specificity and Off-Target Effects | Achieving precise targeting of diseased cells while avoiding healthy tissues remains a significant challenge. |
| Many targeted drug carriers rely on ligand-receptor interactions, but heterogeneity in receptor expression among patients and within tumors can limit efficacy. | |
| Off-target interactions can lead to immune responses, toxicity, or unintended cellular uptake, reducing the overall safety profile of the therapy. | |
| Manufacturing, Scalability, and Regulatory Approval | The complexity of manufacturing nanocarriers, liposomes, polymer-based systems and biologics presents challenges for scalability and cost-effective manufacturing. |
| Stringent regulatory requirements make it difficult for new targeted drug delivery technologies to reach the market, requiring extensive clinical validation and long-term safety studies. | |
| Reproducibility in large-scale production and maintaining batch-to-batch consistency remain significant concerns, particularly for advanced drug formulations. | |
| Drug Loading Capacity and Controlled Release | Many nanocarriers and polymeric delivery systems have limited drug loading capacity, which limits the amount of therapeutic cargo they can deliver. |
| Controlled drug release mechanisms must ensure a balance between rapid drug availability and sustained therapeutic effects. | |
| Some delivery systems can experience premature drug leakage, resulting in reduced therapeutic efficacy and potential toxicity. | |
| Immunogenicity and Long-Term Safety | Foreign nanoparticles and biologic drug carriers can induce immune responses, leading to rapid clearance or undesirable side effects. |
| Lipid nanoparticles (LNPs), exosomes, and viral vectors can induce immune activation, requiring strategies to improve biocompatibility and long-term tolerability. | |
| Chronic administration of nanocarriers may lead to accumulation in tissues such as the liver, spleen, or kidneys, raising concerns about long-term toxicity and clearance. |
Future Directions of Targeted Drug Delivery Systems
| Development of Smarter and Adaptive Nanocarriers | The next generation of drug delivery systems will incorporate stimuli-responsive materials to release drugs only in the presence of specific physiological cues (e.g., pH changes, enzyme activity, temperature changes). |
| Smart nanocarriers will use biosensors and AI-driven design to improve drug targeting and optimize dosing in real time. | |
| Cell membrane-coated nanoparticles (e.g., red blood cell-mimicking nanocarriers) will be explored for enhanced immune evasion and prolonged circulation time. | |
| Advancements in Gene and RNA-Based Therapeutics | Targeted delivery is essential for CRISPR/Cas9 gene-editing therapies, mRNA vaccines, and siRNA therapeutics. Continued research will focus on optimizing delivery vehicles, such as lipid nanoparticles (LNPs), polymeric micelles, and virus-like particles. |
| The combination of gene editing and nanotechnology could pave the way for personalized medicine, where therapies are tailored to a patient's genetic profile. | |
| Integration with Artificial Intelligence (AI) and Machine Learning | AI-driven drug delivery models will help predict the optimal nanocarrier composition, dosing regimen, and patient-specific treatment strategies. |
| Machine learning algorithms can analyze biological data to improve the precision and efficiency of drug targeting mechanisms. | |
| AI-guided formulation development will reduce trial-and-error approaches and accelerate the translation of new therapies into clinical practice. | |
| Enhancing Drug Delivery to Hard-to-Reach Tissues | Overcoming the blood-brain barrier (BBB) remains a priority for the treatment of neurological disorders such as Alzheimer's, Parkinson's and brain tumors. |
| Emerging techniques, including ultrasound-triggered drug release, peptide-based nanocarriers, and exosome-mediated transport, show potential for effective CNS drug delivery. | |
| Inhalable nanoparticle systems are being investigated for pulmonary drug delivery in respiratory diseases such as cystic fibrosis, tuberculosis, and lung cancer. | |
| Personalized and Precision Medicine Approaches | The future of drug delivery lies in tailored therapies that take into account a patient's unique genetic, epigenetic and metabolic profiles. |
| Personalized medicine will involve the customization of drug carriers based on patient-specific biomarkers to maximize efficacy and minimize side effects. | |
| 3D bioprinting and organ-on-a-chip technologies will be used to test targeted delivery systems in patient-derived models, improving the efficiency of drug development | |
| Sustainable and Biodegradable Drug Carriers | The shift towards biodegradable and non-toxic nanomaterials will reduce concerns about long-term accumulation of nanoparticles in the body. |
| Natural polymers (e.g., hyaluronic acid, chitosan, alginate) and lipid-based delivery systems will continue to be explored for their biocompatibility and safety advantages. | |
| Green nanotechnology will aim to reduce the environmental footprint of nanoparticle production while ensuring scalable manufacturing techniques. |
Creative Biostructure offers comprehensive liposome services, exosome services and analysis of drug delivery systems, to advance your drug delivery research, contact us today to learn more.
References
- Ezike TC, Okpala US, Onoja UL, et al. Advances in drug delivery systems, challenges and future directions. Heliyon. 2023;9(6):e17488. doi:10.1016/j.heliyon.2023.e17488
- Gagliardi A, Giuliano E, Venkateswararao E, et al. Biodegradable polymeric nanoparticles for drug delivery to solid tumors. Front Pharmacol. 2021;12. doi:10.3389/fphar.2021.601626
- Jain KK. An overview of drug delivery systems. In: Jain KK, ed. Drug Delivery Systems. Vol 2059. Springer New York; 2020:1-54. doi:10.1007/978-1-4939-9798-5_1
- Mi P. Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics. 2020;10(10):4557-4588. doi:10.7150/thno.38069
- Nunes D, Andrade S, Ramalho MJ, Loureiro JA, Pereira MC. Polymeric nanoparticles-loaded hydrogels for biomedical applications: a systematic review on in vivo findings. Polymers. 2022;14(5):1010. doi:10.3390/polym14051010
- Patil SM, Sawant SS, Kunda NK. Exosomes as drug delivery systems: A brief overview and progress update. European Journal of Pharmaceutics and Biopharmaceutics. 2020;154:259-269. doi:10.1016/j.ejpb.2020.07.026
- Shi P, Cheng Z, Zhao K, et al. Active targeting schemes for nano-drug delivery systems in osteosarcoma therapeutics. J Nanobiotechnol. 2023;21(1):103. doi:10.1186/s12951-023-01826-1