EDS Analysis
Energy-Dispersive X-ray Spectroscopy (EDS), also known as EDX or EDXA, is used for elemental analysis and chemical characterization. EDS analysis has become a powerful analytical technique used in various scientific fields, including structural biology. When combined with electron microscopy, EDS enables researchers to delve deep into the elemental composition of biological samples at a microscopic level. This approach provides valuable insights into the presence and distribution of key elements within complex biological systems. In structural biology, understanding these elemental details is crucial. Elements play vital roles in the stability, function, and interactions of macromolecules such as proteins, nucleic acids, and metal-containing biomolecules.
Principles of EDS Analysis
EDS operates on the principle of characteristic X-ray emission. A sample that is bombarded with high-energy electrons in an electron microscope—usually a scanning electron microscope (SEM), or transmission electron microscope (TEM)—absorbs this energy, and the inner-shell electrons of the sample's atoms are ejected. As outer-shell electrons fall to fill these vacancies, they release energy as X-rays. Each element in the periodic table has a unique electronic structure, so the energy of the emitted X-rays is characteristic of specific elements. By detecting and measuring these energies, EDS can identify and quantify the elements present in the sample.
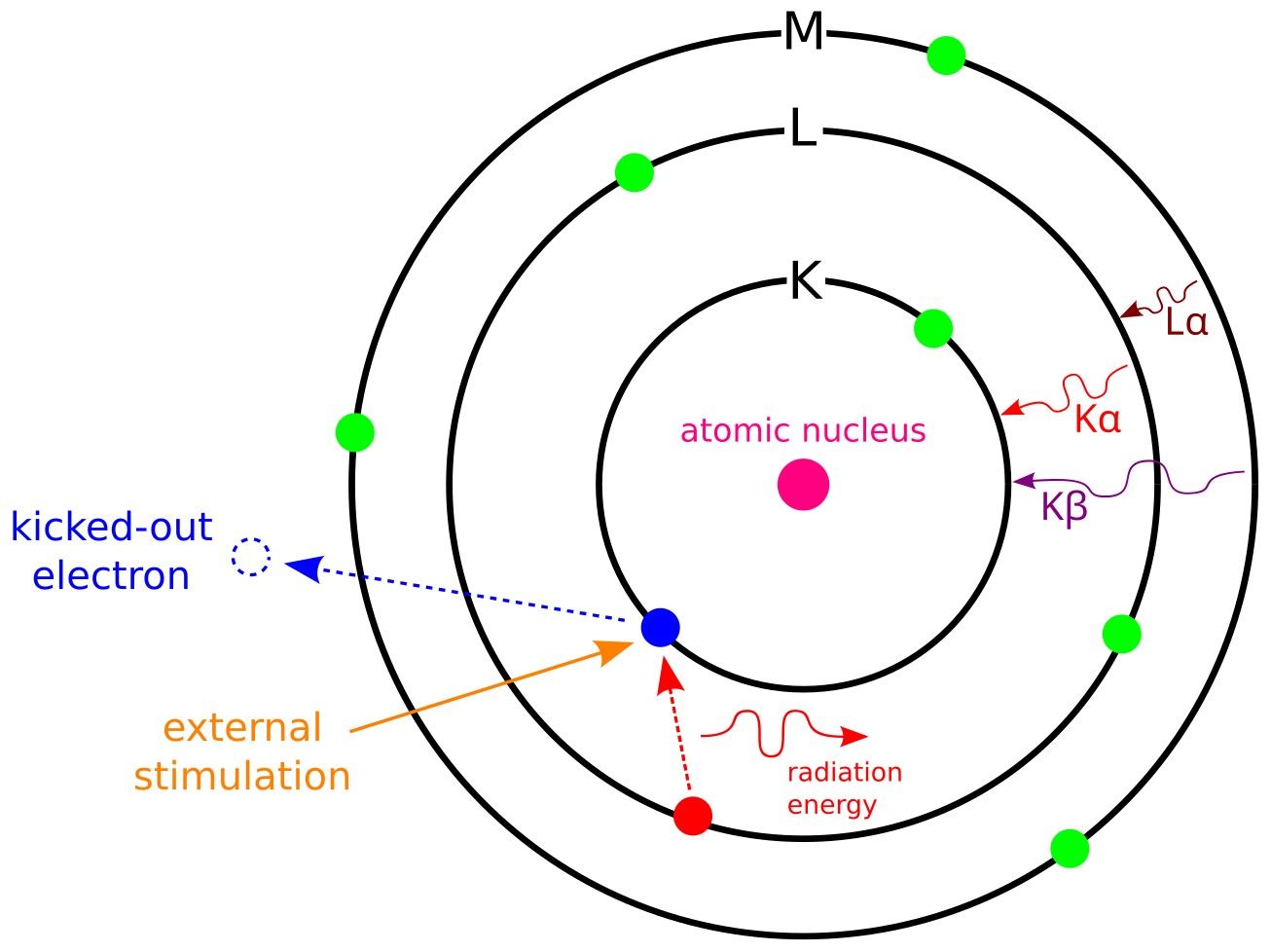 Figure 1: Principle of EDS.
Figure 1: Principle of EDS.
In EDS, X-ray detectors capture these emitted X-rays and convert them into signals, which are then processed to produce an elemental spectrum. The spectrum displays peaks corresponding to specific energy levels, each associated with a particular element. This qualitative and quantitative data allows researchers to analyze the elemental composition of biological samples with high precision. This ability to study element spatial distribution at the nanoscale makes EDS an indispensable tool for advancing our understanding of complex biological structures.
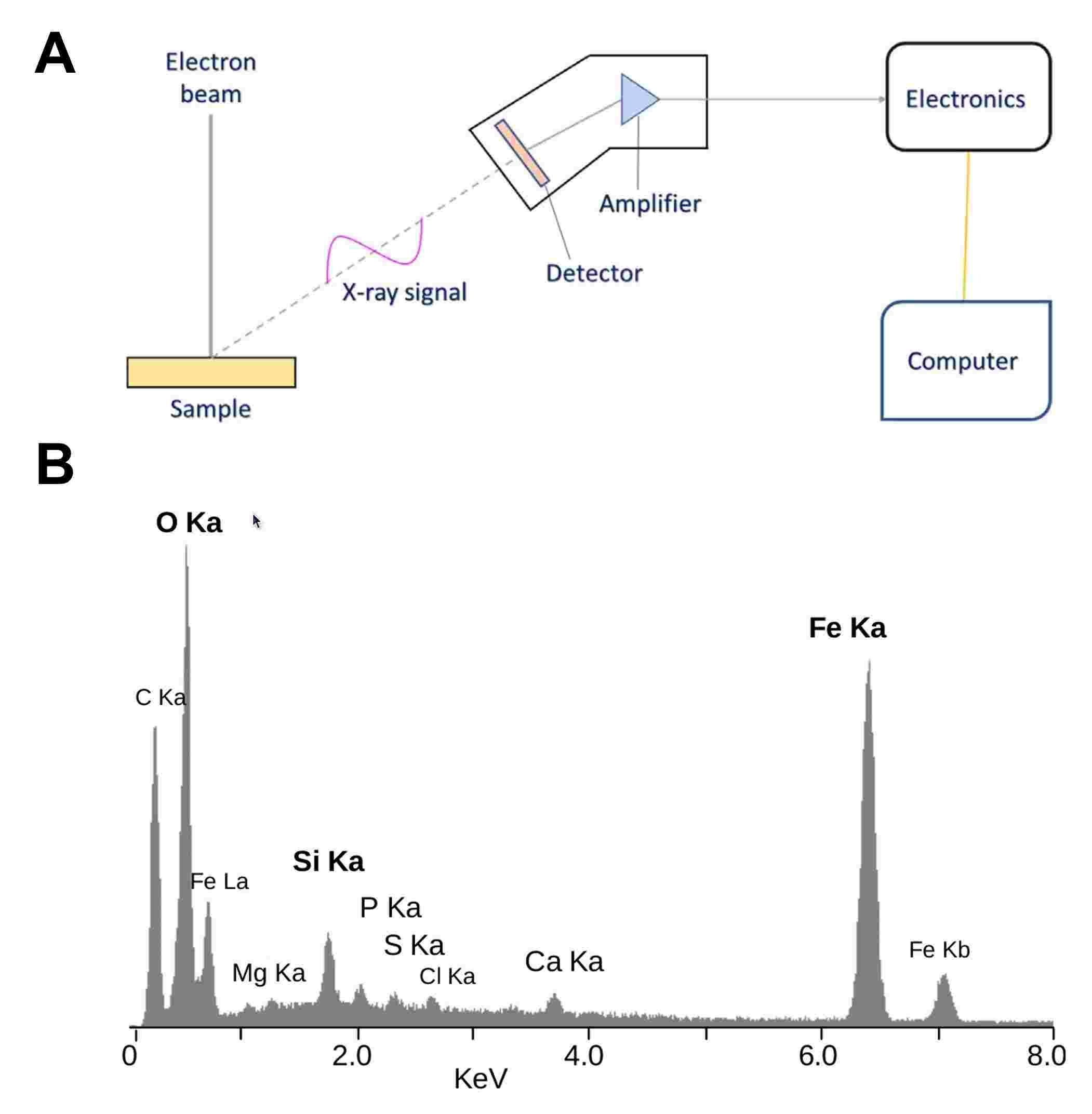 Figure 2: A. Block diagram of energy dispersive X-ray analysis principle (Kumar et al., 2023). B. EDS spectrum of the mineral crust of the vent shrimp Rimicaris exoculate. Most of these peaks are X-rays emitted when electrons return to the K electron shell (K-alpha and K-beta lines). One peak is from the L shell of iron.
Figure 2: A. Block diagram of energy dispersive X-ray analysis principle (Kumar et al., 2023). B. EDS spectrum of the mineral crust of the vent shrimp Rimicaris exoculate. Most of these peaks are X-rays emitted when electrons return to the K electron shell (K-alpha and K-beta lines). One peak is from the L shell of iron.
Workflow in EDS Analysis
The EDS analysis process in structural biology typically involves several steps:
- Sample Preparation: Proper sample preparation is essential for accurate EDS results. Biological samples must be thin enough to allow electron penetration in TEM or SEM. To maintain their structure, samples often require dehydration, cryo-fixation, or resin embedding. Additionally, samples should ideally be free from external contaminants, as these can introduce foreign elements that may interfere with the analysis.
- Electron Microscopy Imaging: The sample is than placed in an electron microscope, where it is scanned with a focused electron beam. High-resolution images are obtained, allowing researchers to pinpoint specific regions for elemental analysis.
- X-ray Detection and Spectral Acquisition: As the electron beam interacts with the sample, it generates characteristic X-rays. These X-rays are captured by an energy-dispersive detector, which converts the X-rays into electronic signals. These signals are then processed into an energy spectrum.
- Data Analysis and Elemental Mapping: The energy spectrum is analyzed to identify peaks corresponding to specific elements. Using specialized software, the data is further processed to create an elemental map. This map shows the distribution of each element within the sample, offering valuable insights into how elements are localized in biological structures.
- Interpretation of Results: In structural biology, the elemental information from EDS is interpreted in the context of the biological function and structure of the sample. For instance, the presence of calcium in a protein complex might suggest a stabilizing role, while phosphorus may indicate nucleic acids or phosphate-rich cellular regions.
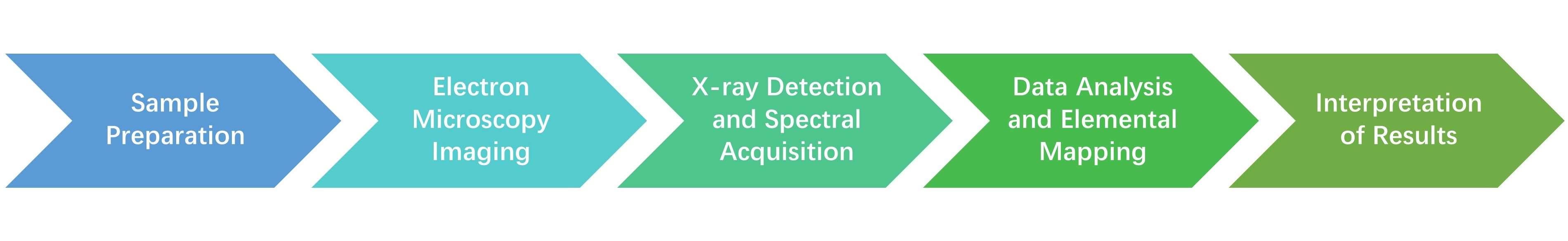 Figure 3: Process of EDS analysis.
Figure 3: Process of EDS analysis.
EDS Analysis in the Context of Structural Biology
Structural biology focuses on exploring the molecular structure and function of biological macromolecules. Techniques like X-ray crystallography, cryo-Electron Microscopy (cryo-EM), and NMR spectroscopy are invaluable for revealing the 3D structures of proteins, nucleic acids, and other macromolecules. However, these methods usually don't provide insights into elemental composition. EDS analysis fills this gap by offering complementary data on elemental distribution within a sample. This is especially important for studying metalloproteins, cofactor interactions, mineralized tissues, and cellular components rich in elements like phosphorus, sulfur, iron, and calcium. Together, these methods give a more complete picture of biological systems.
By combining EDS with high-resolution electron microscopy, structural biologists can study both the architecture and chemical properties of biological structures. For example, in protein complexes, metal ions often act as active sites or stabilizing agents. EDS can pinpoint the exact location of these ions and identify them, elucidating their roles in biochemical pathways. This technique is also invaluable for studying element-rich cellular compartments, like lysosomes. It helps researchers explore processes such as ion transport, biomineralization, and metal homeostasis, providing a deeper understanding of cellular functions.
Applications of EDS Analysis in Structural Biology
EDS analysis offers numerous applications in structural biology, enhancing our understanding of biological systems at the molecular and cellular levels. Some of the key applications include:
Study of Metalloproteins and Enzyme Active Sites
Metalloproteins contain metal ions like iron, copper, zinc, or manganese, which are crucial for their catalytic activity. EDS analysis helps researchers identify and locate these metal ions within protein structures, offering insights into their roles in enzymatic processes. For example, in metalloproteins like hemoglobin and cytochrome c oxidase, EDS can confirm the presence of iron and copper, respectively. This helps clarify their roles in oxygen transport and electron transfer. Understanding the elemental composition and distribution in these proteins is key to uncovering their mechanisms of action.
Characterization of Biomineralization Processes
Biomineralization is the process by which living organisms produce mineralized tissues, such as bones, teeth, and shells. EDS is invaluable for studying the distribution of elements like calcium, phosphorus, and magnesium in these structures. By mapping these elements, researchers can explore how organisms control mineral deposition, which is vital for support, protection, and other biological functions. For example, in bone tissue, EDS can reveal the calcium-to-phosphorus ratio, providing insights into bone density and health.
Analysis of Nucleic Acids and Phosphorylated Compounds
Phosphorus is a key element in nucleic acids (DNA and RNA), and EDS can detect and localize phosphorus-rich regions within cells. This is particularly useful for studying chromatin organization and nuclear structure. EDS can also identify regions with high concentrations of other phosphorylated compounds, such as ATP, in cellular compartments like mitochondria. This provides valuable information on energy metabolism and signaling.
Investigation of Cellular Organelles and Ion Transport
Certain cellular organelles, such as lysosomes and vacuoles, contain high concentrations of specific ions or metals. EDS can map these elements within the organelles, offering insights into cellular storage, ion transport, and homeostasis. For example, high calcium concentrations in mitochondria may indicate their role in calcium storage and regulation.
Understanding Protein-Ligand and Protein-Metal Interactions
In structural biology, it is essential to understand how proteins interact with ligands and metal ions, as these interactions often influence protein stability and function. EDS analysis allows researchers to detect the presence of metals or other elements associated with ligands bound to proteins, offering insights into the binding sites and affinity of protein-ligand interactions. This information is valuable in drug design, where identifying binding sites and understanding metal ion involvement can guide the development of therapeutics.
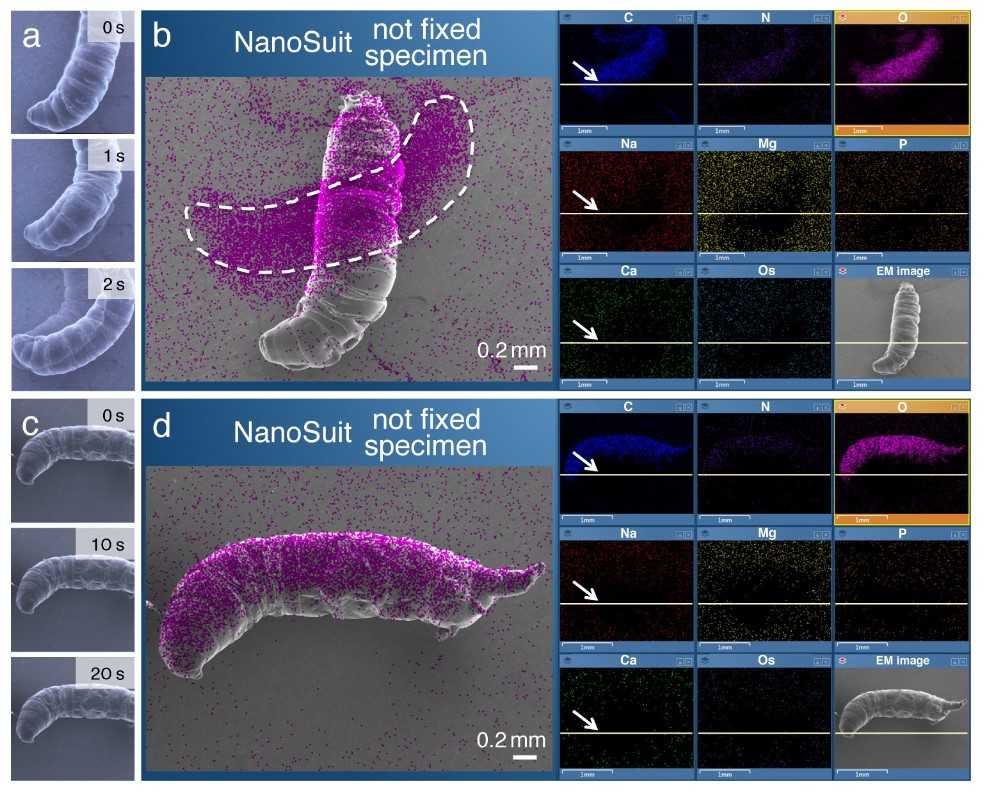 Figure 4: Example of in situ elemental analyses of living biological specimens using EDS method in FE-SEM (Field emission-scanning electron microscope). (a,c) Sequential SEM images of living larvae of Drosophila that underwent electron beam irradiation immediately before the SEM observation. The blurs in (a) are interpreted as the evidence for active movements. (b,d) EDS mapping analysis. The magenta signal indicates the localization of elemental oxygen. Note that the larger area covered by elemental oxygen in (b) is due to the movement (displacement) of the living larva during the measurements. The arrows in (b,d) indicate the current positions of the scanning electron beam. (Takaku et al., 2020)
Figure 4: Example of in situ elemental analyses of living biological specimens using EDS method in FE-SEM (Field emission-scanning electron microscope). (a,c) Sequential SEM images of living larvae of Drosophila that underwent electron beam irradiation immediately before the SEM observation. The blurs in (a) are interpreted as the evidence for active movements. (b,d) EDS mapping analysis. The magenta signal indicates the localization of elemental oxygen. Note that the larger area covered by elemental oxygen in (b) is due to the movement (displacement) of the living larva during the measurements. The arrows in (b,d) indicate the current positions of the scanning electron beam. (Takaku et al., 2020)
Advantages and Limitations of EDS Analysis
The advantages and limitations of EDS analysis are summarized in the table below:
| Advantages of EDS Analysis | |
| High Sensitivity to Elements | EDS can detect a wide range of elements, from light elements like carbon and nitrogen to heavy metals, making it versatile for biological analysis. |
| Non-destructive Analysis | EDS does not require sample destruction, allowing for subsequent analyses or further observation. |
| Compatibility with Electron Microscopy | The integration with SEM and TEM provides both structural and elemental information in a single analysis, offering a comprehensive view of the sample. |
| Limitations of EDS Analysis | |
| Lower Sensitivity for Light Elements | While EDS is highly effective for elements with atomic numbers above sodium, it is less sensitive to lighter elements like hydrogen, carbon, and nitrogen, which are abundant in biological molecules. |
| Requirement of Electron Beam Exposure | Biological samples are sensitive to electron beam damage, which may limit the duration or intensity of exposure and necessitate careful sample preparation. |
| Possible Overlap in Spectral Peaks | EDS spectra can have overlapping peaks, making it challenging to differentiate elements with similar X-ray energies. |
In short, Energy-Dispersive X-ray Spectroscopy (EDS) is a powerful tool in structural biology, helping researchers analyze the elemental composition and distribution of biological samples. From studying metalloproteins and biomineralization to investigating ion transport and protein-metal interactions, EDS has numerous applications that enhance our understanding of biological systems.
At Creative Biostructure, we specialize in a range of advanced structural analysis techniques, including X-ray, Nuclear Magnetic Resonance (NMR), and Mass Spectrometry (MS). Our team is dedicated to providing high-quality, precise data to support your research and development needs. If you are looking for reliable solutions in structural analysis, please explore our services or contact us directly to discuss how we can help elevate your projects. Contact us and let's advance scientific discovery together!
References
- Kumar SP, Balaji D, Mandlimath TR. Characterization of flexible ceramics. In: Advanced Flexible Ceramics. Elsevier; 2023:25-43.
- Takaku Y, Takehara S, Suzuki C, Suzuki H, Shimomura M, Hariyama T. In situ elemental analyses of living biological specimens using 'NanoSuit' and EDS methods in FE-SEM. Sci Rep. 2020;10(1):14574.
