Exosome Research in Human Chronic Myelogenous Leukemia
The discovery of the role of exosomes in cellular interactions has generated much interest in cancer research. Exosomes are small lipid vesicles that deliver molecular cargoes, such as proteins and nucleic acids (RNA and DNA), and are thought to be involved in the diagnosis and prognosis of certain conditions, especially solid tumors.
Emerging evidence suggests that exosomes released by cancer cells can mediate intercellular communication by delivering contents. In the case of leukemia, exosomes can modulate crosstalk between leukemia cells and the bone marrow (BM) microenvironment, alter physiological homeostasis outside the BM, and carry tumor markers. In addition, research has shown that leukemia-derived exosomes can be used as diagnostic, prognostic, and therapeutic biomarkers for hematologic malignancies.
Overview of Chronic Myelogenous Leukemia
Chronic myelogenous leukemia (CML) is a BM proliferative neoplasm. It is characterized by chromosomal rearrangements t(9;22) and BCR-ABL1 oncoprotein production. BCR-ABL1, a constitutively activated tyrosine kinase, is a critical factor in leukemia formation and disease progression and is considered a marker of CML. Currently, tyrosine kinase inhibitors (TKIs) targeting the BCR-ABL1 p210 protein can control the progression of CML. However, numerous studies have shown that although TKIs are effective agents for the treatment of CML, drug resistance limits its therapeutic potential.
What is the Impact of Exosomes on the Pathogenesis of CML?
Recent research has revealed the role of CML-derived exosomes in the activation of signal transduction pathways associated with cancer cell proliferation. Tumor cells secrete a variety of cytokines, growth factors, adhesion molecules, and matrix proteins to provide an appropriate environment for cancer cell growth and survival. Exosomes are thought to be carriers of these molecules into the tumor microenvironment. CML-derived exosomes communicate between CML cells and surrounding BM stromal cells, leading to suppression of osteogenesis and thus promoting CML progression. In addition, research has found that exosomes released by CML cells directly affect endothelial cells, thereby regulating neointimal formation.
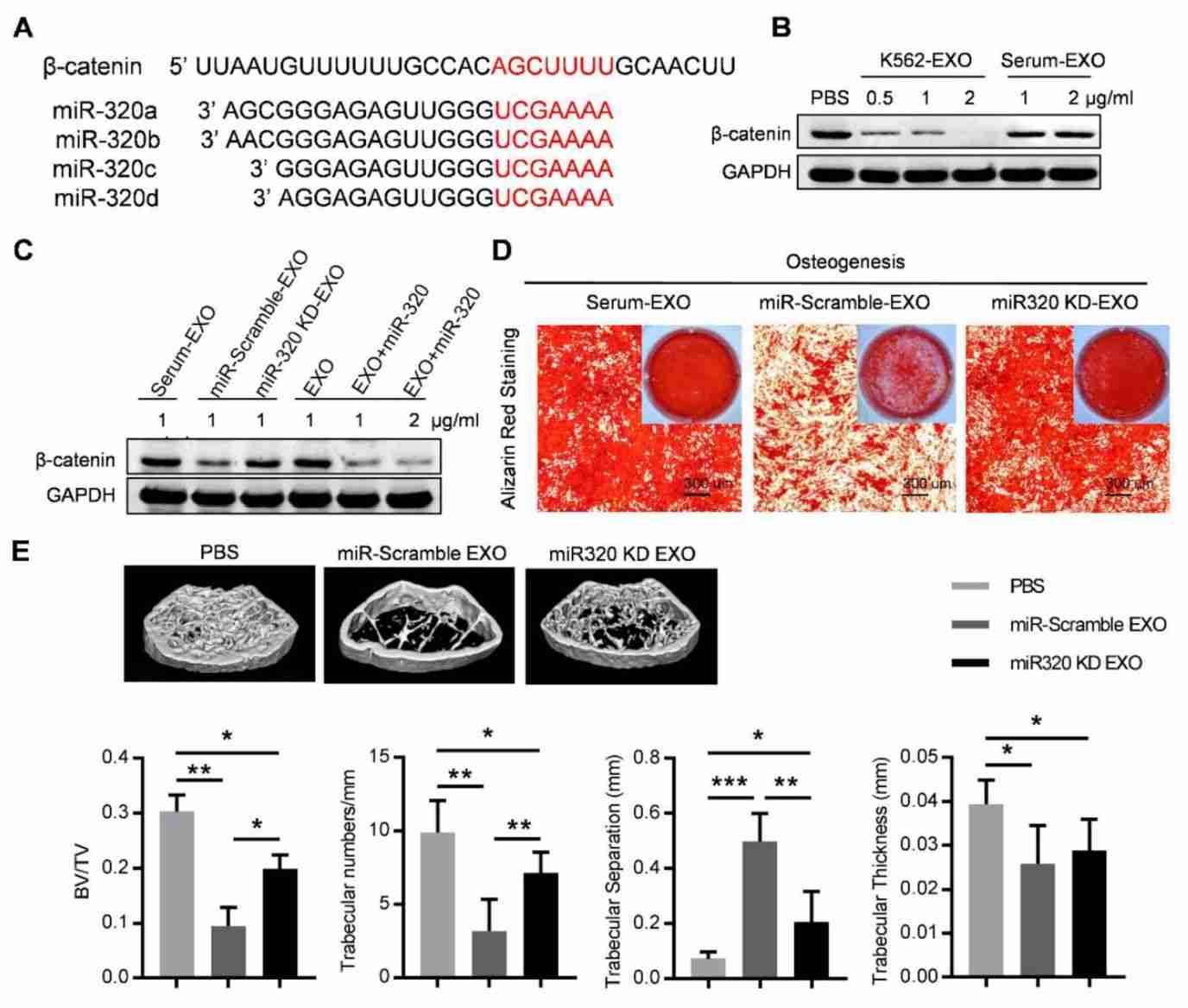 Figure 1. Exosome miR-320 inhibits BMMSC osteogenesis. (Gao X, et al., 2019)
Figure 1. Exosome miR-320 inhibits BMMSC osteogenesis. (Gao X, et al., 2019)
The autocrine action of CML-derived exosomes activates the growth of leukemia cells. Therefore, we provide CML-derived exosomes to help clients explore the role of exosomes in cancer biology, which is essential for proposing innovative therapeutic and biomarker strategies.
| Cat No. | Product Name | Source |
| Exo-CH11 | HQExo™ Exosome-K-562 | Exosome derived from human pleural effusion, leukemia chronic myelogenous (K-562 cell line) |
| Explore All Exosomes Isolated from Human Chronic Myelogenous Leukemia | ||
Exosomes as Carriers of CML Therapeutics
As a strategy to target tumor cells, the use of nucleic acid-based inhibitors of gene expression (e.g., RNAi) has been proposed for the treatment of CML. The ideal delivery system would be able to transport the drug to a specific type of cell, thereby increasing the bioefficacy and decreasing the therapeutic toxicity. Exosomes, which are vesicles 30-150 nm in diameter containing cytokines, growth factors, adhesion molecules, and nucleic acids, are considered new drug delivery tools. Depending on the molecules expressed on the surface, exosomes can target drugs to specific tissues or organs. Research has found that the interleukin 3 receptor (IL3-R) is overexpressed in CML cells and therefore can serve as a target in cancer drug delivery systems. By targeting CML cells with IL3- Lamp2b exosomes loaded with TKI or BCR-ABL siRNA, researchers could inhibit cancer cell growth.
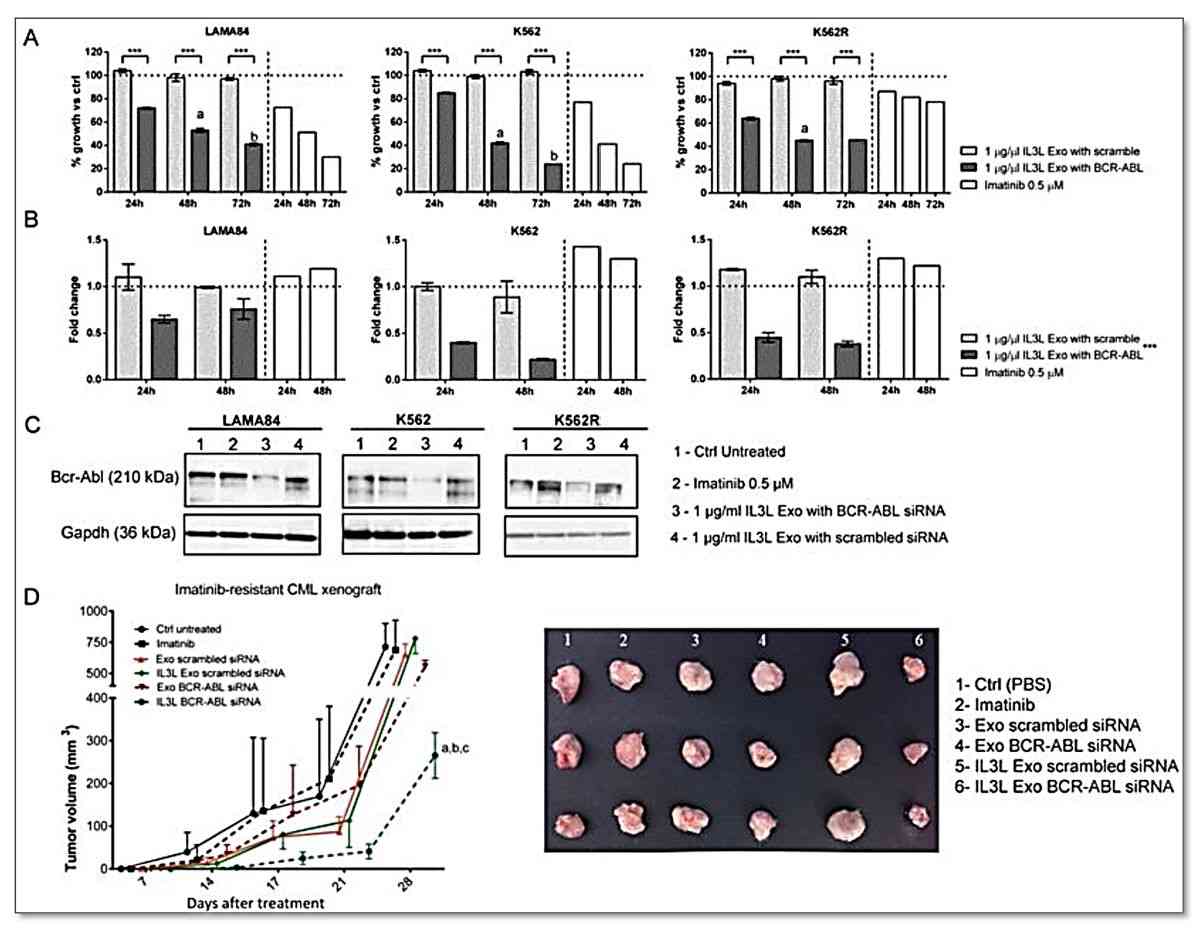 Figure 2. In vitro and in vivo effects of BCR-ABL siRNA loaded IL3L-exosomes. (Bellavia D, et al., 2017)
Figure 2. In vitro and in vivo effects of BCR-ABL siRNA loaded IL3L-exosomes. (Bellavia D, et al., 2017)
Exosomes as Disease Markers in AML
In addition to BCR-ABL1, exosome miRNA levels in AML patients are used for diagnostic, prognostic, and therapeutic monitoring. Research has identified miR155 as a critical player in the pathogenesis of several hematologic malignancies, and AML patients have significantly higher levels of exosome miR155. In addition, statistical analysis showed that exosome miR10b levels are a potential marker for distinguishing AML patients from healthy controls. Another important exosome miRNA is miR532, which has recently been reported to be associated with myeloid malignancies. Although the essential importance of exosome cargo as a biomarker of AML is clear, molecular markers expressed on the surface of exosomes may also be of importance. The heterogeneity of AML can be understood by detecting clusters of CD combinations. These combinations may guide the molecular typing of AML in the clinic.
Creative Biostructure has always and will always support researchers in the search for the best solutions to treat CML. We provide off-the-shelf exosome products that can be used to understand the tumor growth microenvironment, research the action mechanisms of tumor exosomes, and explore new anti-tumor therapies. In addition, our scientists are ready to provide a full suite of services to support exosome-related research, such as exosome isolation, characterization, and customizable exosomes for specific downstream applications. If you have any questions, please feel free to contact us.
References
- Gao X, et al. Chronic myelogenous leukemia cells remodel the bone marrow niche via exosome-mediated transfer of miR-320. Theranostics. 2019. 9(19): 5642-5656.
- Bellavia D, et al. Interleukin 3- receptor targeted exosomes inhibit in vitro and in vivo Chronic Myelogenous Leukemia cell growth. Theranostics. 2017. 7(5): 1333-1345.
- Bernardi S, et al. Extracellular vesicles in the Chronic Myeloid Leukemia scenario: an update about the shuttling of disease markers and therapeutic molecules. Front Oncol. 2024. 13: 1239042.
- Bernardi S, et al. Exosomes in Chronic Myeloid Leukemia: Are We Reading a New Reliable Message?. Acta Haematol. 2020. 143(5): 509-510.
- Taverna S, et al. Role of exosomes released by chronic myelogenous leukemia cells in angiogenesis. Int J Cancer. 2012. 130(9): 2033-2043.