Structural Research of Isomerases
X-ray diffraction technology can be used to determine and understand the structure of isomerase, which enables researchers to study the molecular mechanism and reaction pathway of proteins. In addition, structural biologists can also use the structure of isomerase to design new drugs to treat diseases.
RPE65 protein is the source of isomerase activity in retinal pigment epithelial cells, responsible for maintaining visual function in vertebrates. In 2009, researchers first determined the crystal structure of natural bovine RPE65 with a resolution of 2.14 Å. The basic RPE65 structural motif is a seven-blade β propeller with a single-strand extension on blades VI and VII, and a double-strand extension on blade III.
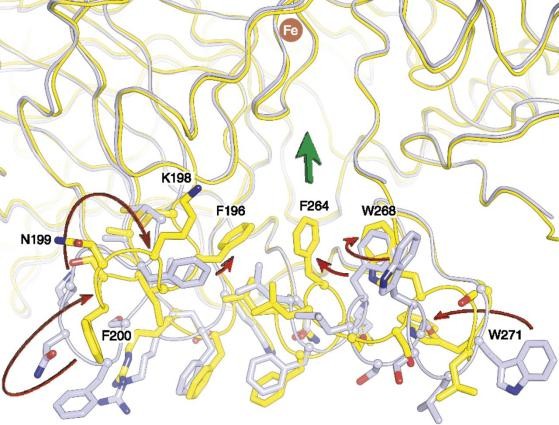 Figure 1. Conformational differences in the membrane-binding surface and active-site entrance observed between lipid-embedded and
delipidated RPE65. (KISER, et al., 2012)
Figure 1. Conformational differences in the membrane-binding surface and active-site entrance observed between lipid-embedded and
delipidated RPE65. (KISER, et al., 2012)
In 2012, the experimental team analyzed the crystal structure and dimer structure of RPE65 in a membrane-like environment and revealed the roles of phospholipids and iron in catalysis. The researchers ultimately proved that the iron cofactor of RPE65 mainly exists in a divalent oxidized state. Iron carboxylate complexes may be intermediates formed during isomerization reactions.
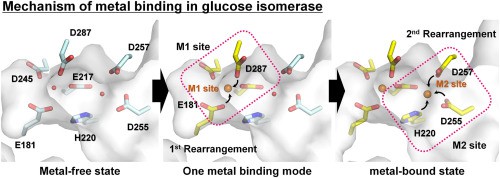 Figure 2. Mechanism of metal binding in glucose isomerase. (NAM, 2021)
Figure 2. Mechanism of metal binding in glucose isomerase. (NAM, 2021)
Glucose/xylose isomerase is two very important isomerases. They not only participate in sugar metabolism but also have industrial applications. Researchers have used the X - crystallography structure of various glucose isomerases to reveal the binding configurations of different substrates and their molecular mechanisms.
In 2021, scientists made efforts to clarify the metal binding mechanism required for glucose isomerase to participate in isomerization. They characterized the crystal structure of Streptomyces rubrum glucose isomerase (SruGI) in both metal-bound and metal-free states at resolutions of 1.4 Å and 1.5 Å, respectively. In the presence of metal, Mg2+ binds to M1 and M2 sites, while in the absence of metal, these sites are occupied by water molecules.
| Protein | Organism | Method | Resolution | PDB Entry ID |
| RPE65 visual cycle retinoid isomerase | Bos taurus | X-ray diffraction | 2.14 Å | 3FSN |
| RPE65 visual cycle retinoid isomerase in a lipid matrix | Bos taurus | X-ray diffraction | 2.50 Å | 4F3D |
| Crystal form A | Bos taurus | X-ray diffraction | 3.00 Å | 4F2Z |
| Crystal form B, iridium derivative | Bos taurus | X-ray diffraction | 2.60 Å | 4F3A |
| Crystal form B, native | Bos taurus | X-ray diffraction | 3.15 Å | 4F30 |
Table 1. Structural research of isomerases.
Creative Biostructure focuses on providing high-quality X-ray crystallography services and providing technical support for research in the field of life science. X-ray crystallography is an effective tool to measure the molecular structure of biomolecules through diffraction patterns, revealing the unique advantages of three-dimensional structure and function of biomolecules.
X-ray crystallography has important application value in analyzing complex biomolecules such as membrane proteins. We use high-intensity X-ray and precise detectors to obtain high-resolution structural data, ensuring that you obtain the most reliable and accurate results. Our professional team will work with you to customize the most suitable solution for your research needs. We are committed to providing you with the best services to help you make further breakthroughs in the field of biomolecular research.
References
- KISER P D, et al. Structure of RPE65 isomerase in a lipidic matrix reveals roles for phospholipids and iron in catalysis. Proceedings of the National Academy of Sciences, 2012, 109(41).
- KISER P D, et al. Crystal structure of native RPE65, the retinoid isomerase of the visual cycle. Proceedings of the National Academy of Sciences, 2009, 106(41): 17325–17330.
- NAM K H. Crystal structure of the metal-free state of glucose isomerase reveals its minimal open configuration for metal binding. Biochemical and Biophysical Research Communications, 2021, 547: 69–74.