Structural Research of Nucleobase-Cation-Symport-1 (NCS1) Family
The nucleobase-cation-symport-1 (NCS1) family is derived from Gram-positive and Gram-negative bacteria, fungi, archaea and plants. The NCS1 family contains more than 2,500 members in the protein database. NCS1 is a H + / Na + transporter of nucleobases and metabolites, including purines and pyrimidines. NCS1 proteins from fungi and plants are classified into two distinct types, Fur and Fcy.
Research Progress of Mhp1 Structure
The first NCS1 protein with a high-resolution structure is Mhp1. Mhp1 consists of 12 transmembrane A-helices (TM1-TM12), 10 of which are arranged into two inverted repeats of five helices. Outward and inward cavities are symmetrically arranged on both sides of the membrane. The outward opening cavity is combined with the base to form an occlusive conformation. Opening and closing of these cavities are controlled by inverted repeat helices 3 and 8. Both the N and C terminal of Mhp1 are located on the cytoplasmic side of the membrane.
Molecular Mechanism of NCS1 Protein Described by Mhp1
Mhp1 undergoes a conformational transition first. The outward conformation changes to a blocked state. The outward cavity consists of TMs 1, 3, 6, 8, and 10. The N-terminal of TM10 folds over the substrate when it binds to it, closing the outward cavity. During the transition from the closed state to the inward state, TMs 3,4,8, and 9 rotate 30° relative to the bundle and shift 3 Å toward the membrane plane. Its movement coincides perfectly with the C-terminal of TM3 and TM9 and the small extracellular spiral outward cavity between TM7 and TM8. At the same time, the movement of TM4 and TM8 causes the sodium ion and substrate binding site to open to the cytoplasm, forming an inward cavity.
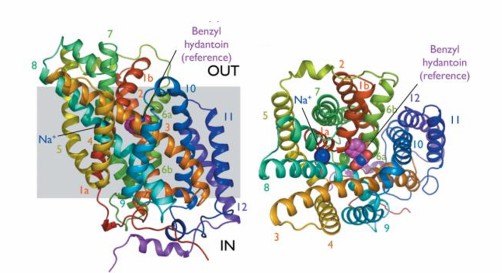 Figure 1. Mhp1 structure on the membrane plane and from outside the membrane. (Weyand S, et al.2008)
Figure 1. Mhp1 structure on the membrane plane and from outside the membrane. (Weyand S, et al.2008)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Mhp1, a nucleobase-cation-symport-1 family transporter | Microbacterium liquefaciens | X-ray diffraction | 2.85 Å | 2JLN |
| Inward facing conformation of Mhp1 | Microbacterium liquefaciens | X-ray diffraction | 3.8 Å | 2X79 |
| CodB, a cytosine transporter in an outward-facing conformation | Proteus vulgaris | X-ray diffraction | 2.4 Å | 7QOA |
| Mhp1, a nucleobase-cation-symport-1 family transporter, in a closed conformation with indolylmethyl-hydantoin | Microbacterium liquefaciens | X-ray diffraction | 3.4 Å | 4D1A |
| Mhp1, a nucleobase-cation-symport-1 family transporter, in a closed conformation with benzyl-hydantoin | Microbacterium liquefaciens | X-ray diffraction | 3.8 Å | 4D1B |
| Mhp1, a nucleobase-cation-symport-1 family transporter, in a closed conformation with bromovinylhydantoin bound | Microbacterium liquefaciens | X-ray diffraction | 3.7 Å | 4D1C |
| Mhp1, a nucleobase-cation-symport-1 family transporter with the inhibitor 5-(2-naphthylmethyl)-l-hydantoin | Microbacterium liquefaciens | X-ray diffraction | 3.7 Å | 4D1D |
Table 1. Structural research of mammalian cell entry (MCE) proteins.
In recent years, an increasing number of NCS1 structures have been analyzed by X-ray crystallography. To explore its important role in transporting nucleobases and metabolites.
If you want to explore NCS1 structures by X-ray diffraction. Creative Biostructure is your perfect solution. We have long been committed to the study of structural biology and membrane proteins and have rich experience.
In addition to the structural determination of membrane proteins, we can also analyze nucleic acids, ribosomes, small proteins, protein complexes, protein-ligand complexes and viruses. If you are interested in our services, please contact us for more details.
References
- Weyand S, et al.Structure and molecular mechanism of a nucleobase-cation-symport-1 family transporter. Science. 2008;322(5902):709-713.
- Patching, S.G. Recent developments in nucleobase cation symporter-1 (NCS1) family transport proteins from bacteria, archaea, fungi and plants. J Biosci.2018,797–815.
