Structural Research of Flagellar Motor Proteins
Bacterial flagella are motile organelles responsible for bacterial cells' rapid movement to a more ideal environment. Flagellar motor proteins are key components that drive bacterial flagella rotation. Flagellar motor proteins include several components, such as flig, flim, Flin, etc. These proteins regulate flagella rotation direction and speed by forming a complex interaction network.
The structural study of flagellar motor proteins is of substantial significance for revealing bacteria's movement mechanism, understanding the working principle of flagellar motors, and developing effective antibacterial drugs. By studying flagellar motor proteins' structure, we can understand their regulatory mechanism and interaction with other proteins. This will provide new targets and strategies for antibacterial drug development. In addition, it also helps to reveal the significant role of bacterial movement in infection. It provides new clues for the treatment and control of the disease.
Fill-c forms a partial ring in the crystal, and the full ring model based on the crystal structure is consistent with the ring shape observed in situ. The study shows that each fill ring is coaxially sandwiched between the MOTA ring and the dimeric periplasmic MOTB portion of the stator unit. The density of the central pore of the fill ring is consistent with the plug / connector region extended by MOTB in an active conformation.
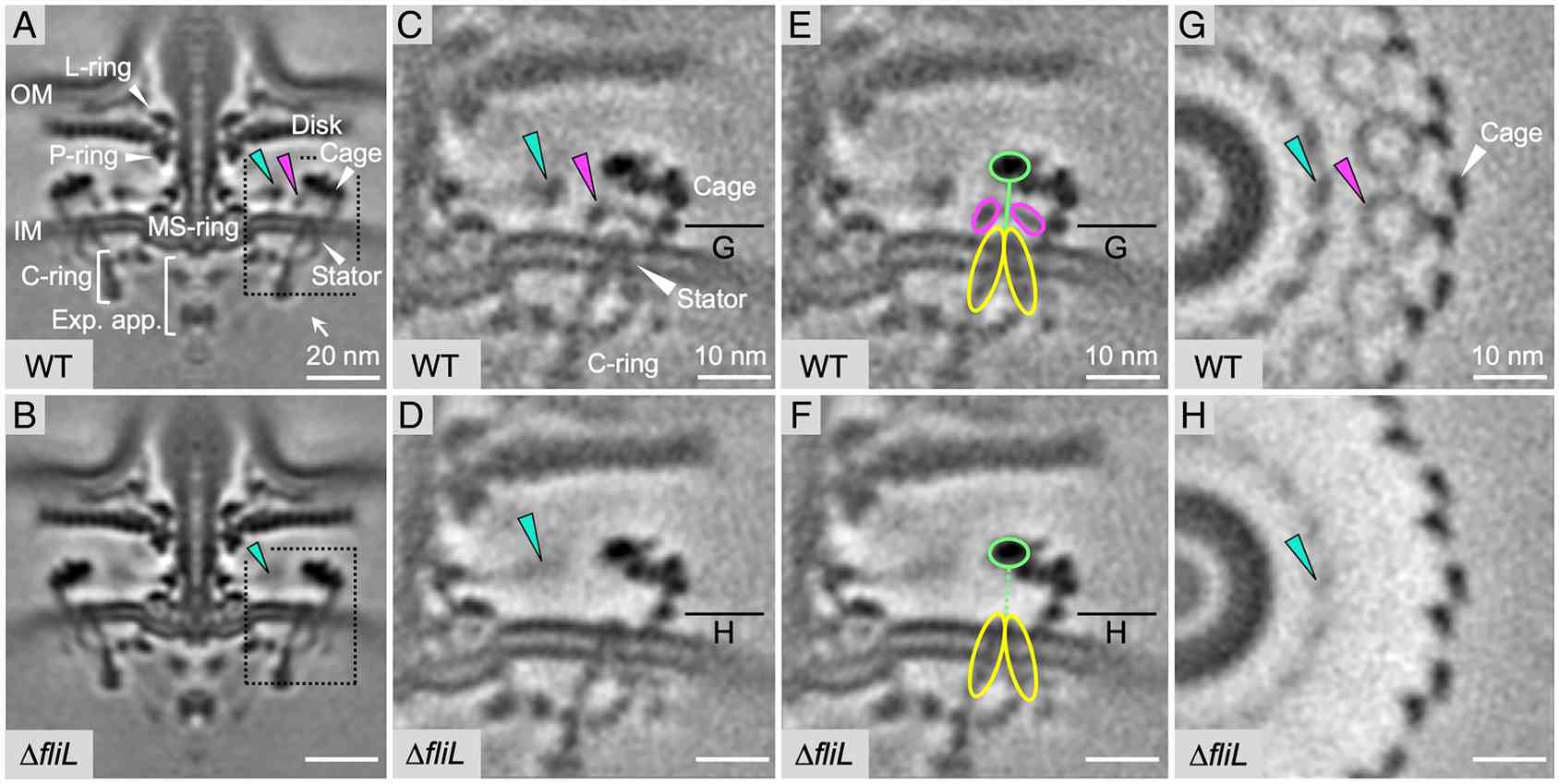 Figure 1. Structures of intact H. pylori flagellar motor in WT and fliL mutant cells. (Tachiyama S, et al., 2022)
Figure 1. Structures of intact H. pylori flagellar motor in WT and fliL mutant cells. (Tachiyama S, et al., 2022)
| Protein | Organism | Method | Resolution | PDB Entry ID |
|---|---|---|---|---|
| MotAB stator units: Campylobacter jejuni | Campylobacter jejuni subsp. jejuni 81-176 | Cryo-EM single particle analysis | 3.10 Å | 6YKM |
| MotAB stator units: Clostridium sporogenes | Clostridium sporogenes | Cryo-EM single particle analysis | 3.40 Å | 6YSF |
| MotAB stator units: Bacillus subtilis | Bacillus subtilis subsp. subtilis str. 168 | Cryo-EM single particle analysis | 3.50 Å | 6YSL |
| flagellar LP ring: Salmonella enterica | Salmonella enterica subsp. enterica serovar Typhimurium | Cryo-EM single particle analysis | 3.50 Å | 7CLR |
Table 1. Structural research of flagellar motor proteins.
Creative Biostructure is a biotechnology company specializing in protein structure research. For scientific research and industrial applications, we provide flagellar motor protein structural analysis services. We have an experienced team, usually combining protein mutagenesis, cryo-electron tomography (cryo-ET) and crystallography to reveal its protein structure. At the same time, we also provide services such as protein complex modeling, protein engineering and drug design for flagellar motor proteins. We are committed to offering customers high-quality data and solutions to help you make breakthroughs in flagellar motor protein research. Whether you are engaged in academic research or drug research and development, we will become your reliable partner. If you are interested in our services, you can contact us at any time to learn more about flagellar motor proteins and protein structure research.
Reference
- Tachiyama S, et al. The flagellar motor protein FliL forms a scaffold of circumferentially positioned rings required for stator activation. Proc Natl Acad Sci U S A. 2022. 119 (4).
