Structural Research of Phosphoinositide Kinases
Phosphoinositide kinases are therapeutic targets for a variety of human diseases, including cancer, viral infections, neurodegenerative diseases, developmental disorders, diabetes, and inflammatory diseases. Therefore, understanding how phosphatidylinositol kinases are regulated is critical for the treatment of many devastating human diseases. Recent structural studies of phosphatidylinositol kinases have revealed unique molecular mechanisms for their regulation. Several mutations occur in phosphatidylinositol kinases that may contribute to disease development by causing excessive activation or inactivation of the kinase. Phosphatidylinositol kinase is a therapeutic target for a variety of human diseases, including cancer, viral infections, neurodegenerative diseases, developmental disorders, diabetes, and inflammatory diseases.
Phosphatidylinositol kinases can be divided into three families: One family contains all phosphoinositide 3-kinases (PI3K; also known as phosphatidylinositol 3-kinase) and type III PI4K; another family contains PIKfyve, which is conserved from yeast to humans and is the only eukaryotic protein that catalyzes the production of phosphatidylinositol 3-kinase (PI3P) to phosphatidylinositol 3,5-bisphosphate (PI (3, 5) P2). PI3P, the substrate of PIKfyve, is produced by class III PI3K VPS34. In addition, the main source of PI5P in cells is also closely related to PIKfyve and is produced indirectly, mainly by dephosphorylation of PI (3, 5) P2. The PIKfyve complex is localized to endosomal membranes. In endosomes/lysosomes, PI (3, 5) P2 is involved in the regulation of ion channel activity and plays a crucial role in ion concentration homeostasis.
In mammalian cells, PIKfyve consists of 2098 amino acids and contains four putative structural domains: the FYVE structural domain that binds to PI3P, the CCT structural domain that mediates the interaction with Vac14, the CCR module that is currently not structurally visible, and a kinase structural domain with sequence homology to PI5P4K and PI4P5K. In addition to lipid kinase activity, PIKfyve has protein Kinase activity is regulated by inhibitory autophosphorylation. Deletion of the PIKfyve protein is embryonically lethal in mammals.
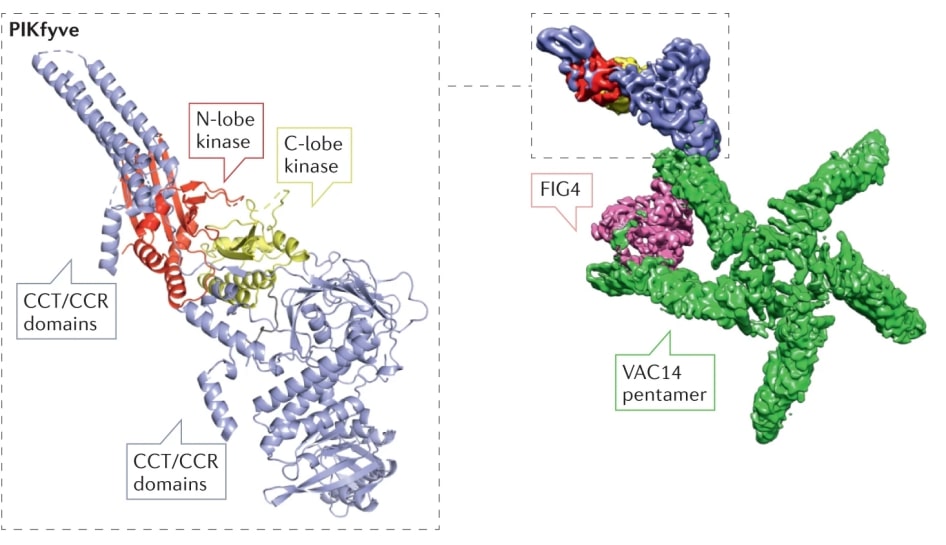 Figure 1. Schematic diagram of the PIKfyve structure. (Burke JE, et al., 2023)
Figure 1. Schematic diagram of the PIKfyve structure. (Burke JE, et al., 2023)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Phosphatidylinositol 4-kinase IIα: Homo sapiens E (expressed in E. coli) | Homo sapiens, Tequatrovirus T4 | X-ray diffraction | 2.77 Å | 4PLA |
| Akt1 serine / threonine protein kinase: Homo sapiens E (expressed in Spodoptera frugiperda) | Homo sapiens, Danio rerio, Lama glama | X-ray diffraction | 2.05 Å | 7APJ |
Table 1. Structural research of phosphoinositide kinases.
Structural studies of phosphatidylinositol kinase proteins involve a variety of structural biology methods and techniques. Examples include X-ray crystallography, cryo-electron microscopy (Cryo-EM), and nuclear magnetic resonance (NMR) can be used to study the structure.
Creative Biostructure can provide protein structure analysis services using X-ray crystallography, cryo-EM, and NMR spectroscopy. This allows you to obtain timely information on proteins' 3D structure and understand their possible functions and interactions. We can help you analyze protein structural data and present them using visualization tools. This will help you understand the structural features, catalytic mechanisms, and possible drug targets of the proteins.
Our team includes experienced biochemists or structural biologists who can provide expert advice and answers to a wide range of protein structure studies. You can ask questions, seek advice on experimental design, or discuss issues related to the field. If you are interested in our services, please contact us for a more detailed description of our services.
References
- Burke JE, et al. Beyond PI3Ks: targeting phosphoinositide kinases in disease. Nat Rev Drug Discov. 2023; 22 (5): 357-386.
- Burke JE. Structural basis for regulation of phosphoinositide kinases and their involvement in human disease. Mol Cell. 2018; 71 (5): 653-673.