Structural Research of Solute Carrier (SLC) Transporter Superfamily
The solute carrier (SLC) superfamily currently includes 458 transport proteins from 65 families that carry a wide range of substances across cell membranes. Human SLCs transport nutrients, metabolites, exogenous substances, and drugs, including inorganic ions, amino acids, sugars, neurotransmitters, lipids, and drugs. Most SLCs function as coupled co-transporter proteins that utilize the H+ or Na+ gradient as a driving force for the transport of substrates against the concentration gradient into the cell, and play essential roles in various physiological and pharmacological processes.
SLC's function in health and disease
As one of the major regulators of cellular substance transport, SLC proteins are associated with a wide range of cellular and physiological processes. Some SLC proteins are tissue-specific, such as the SLC6 and SLC18 family members, which regulate the concentration of neurotransmitters in synapses. Many SLCs are also involved in the transport of essential nutrients across selective barriers between tissues, such as the blood-brain barrier or intestinal epithelium. Some SLCs act as "receptors" and participate in intracellular signaling systems. For example, SLC30A8, a zinc transporter protein closely related to diabetes, is highly expressed in pancreatic cell membranes. In addition, some SLC proteins contain viral binding sites that facilitate viral entry into the cell.
Advances in structural research of the SLC family
SLC proteins contain between 1 and 16 transmembrane (TM) domains, although most tend to contain 7 and 12 TM domains. However, most SLC transporter proteins share some common structural features, namely the pseudo-symmetry of the core TM domains. The two most common structural folds in SLC proteins are the major facilitator superfamily (MFS) and the leucine transporter protein (LeuT)-like folds. The MFS fold consists of a pseudo-repeat of two six TM helices linked by a cytoplasmic loop, and the LeuT fold consists of two 5-TM helices, each containing a bundle and a scaffolding domain. The MFS fold consists of two pseudo repeats of 6-TM helices connected by a cytoplasmic loop, and the LeuT fold consists of two 5-TM helices, each containing a bundle and a scaffold domain.
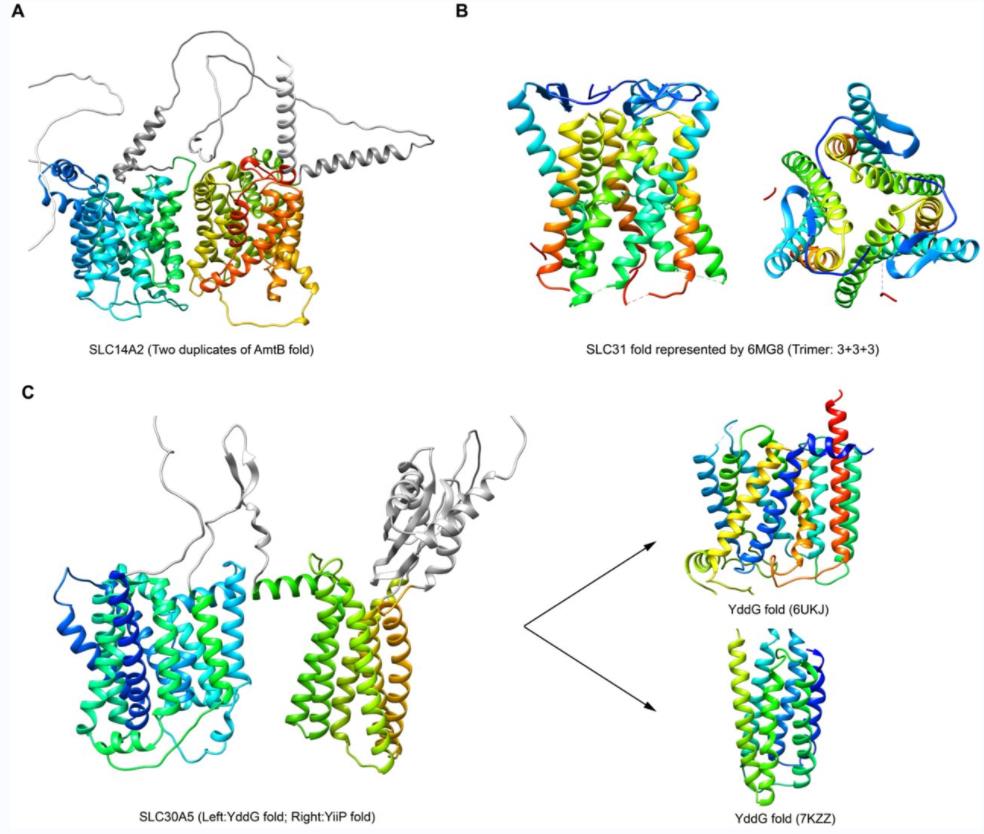 Figure 1. Folds of SLC14A2, SLC31, and SLC30A5. (Xie T, et al., 2022)
Figure 1. Folds of SLC14A2, SLC31, and SLC30A5. (Xie T, et al., 2022)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Choline transporter-like protein 1 | Homo sapiens | Cryo-EM single particle analysis | 3.86 Å | 7WWB |
| LaINDY crystallized in the presence of alpha-ketoglutarate and malate | Lactobacillus acidophilus | X-ray diffraction | 2.86 Å | 6WTW |
| LaINDY-alpha-ketoglutarate complex | Lactobacillus acidophilus | Cryo-EM single particle analysis | 3.71 Å | 6WU4 |
| VcINDY-Na+ in amphipol | Vibrio cholerae | Cryo-EM single particle analysis | 3.16 Å | 6WU3 |
| LaINDY-malate complex | Lactobacillus acidophilus | Cryo-EM single particle analysis | 3.36 Å | 6WU2 |
| Apo LaINDY | Lactobacillus acidophilus | Cryo-EM single particle analysis | 3.09 Å | 6WU1 |
| Inhibited I (Chloride + Salicylate) | Tursiops truncatus | Cryo-EM single particle analysis | 3.8 Å | 7S9A |
| Inhibited II (Sulfate +Salicylate) state | Tursiops truncatus | Cryo-EM single particle analysis | 3.7 Å | 3S9E |
| Sensor Up (compact) state | Tursiops truncatus | Cryo-EM single particle analysis | 3.3 Å | 7S8X |
| Sensor Down I (Expanded) state | Tursiops truncatus | Cryo-EM single particle analysis | 4.2 Å | 7S9B |
| Sensor Down II (Expanded II) state | Tursiops truncatus | Cryo-EM single particle analysis | 6.7 Å | 7S9C |
| Intermediate state | Tursiops truncatus | Cryo-EM single particle analysis | 4.6 Å | 7S9D |
| VcINDY in complex with terephthalate | Vibrio cholerae | X-ray diffraction | 3.92 Å | 6WTX |
| A bacterial cationic amino acid transporter (CAT) homologue bound to Arginine. | Escherichia coli K-12 | X-ray diffraction | 3.13 Å | 6F34 |
| VcINDY-Na-Fab84 in nanodisc | Homo sapiens | Cryo-EM single particle analysis | 3.15 Å | 6WW5 |
| The sodium-dependent phosphate transporter | Thermotoga maritima MSB8 | X-ray diffraction | 2.302 Å | 6L85 |
| HsPepT1 bound to Ala-Phe in the outward-facing occluded conformation | Homo sapiens | Cryo-EM single particle analysis | 4.1 Å | 7PMW |
| HsPepT1 bound to Ala-Phe in the outward-facing open conformation | Homo sapiens | Cryo-EM single particle analysis | 3.5 Å | 7PMX |
| Apo HsPepT1 in the outward facing open conformation | Homo sapiens | Cryo-EM single particle analysis | 3.9 Å | 7PN1 |
| murine Solute Carrier 26 family member A9 (Slc26a9) anion transporter in an intermediate state | Mus musculus | Cryo-EM single particle analysis | 7.77 Å | 6RTF |
| Proton symporter in the inward open form | Escherichia coli | X-ray diffraction | 2.915 Å | 6E9N |
| Proton symporter mutant E133Q in the outward substrate-bound form | Escherichia coli | X-ray diffraction | 3.501 Å | 6E9O |
| Peptide transporter DtpA-nanobody in complex with valganciclovir | Escherichia coli | X-ray diffraction | 2.645 Å | 6GS4 |
| Divalent anion/Na+ symporter and a humanized variant | Vibrio cholerae O1 biovar El Tor str. N16961 | X-ray diffraction | 2.8 Å | 5UL7 |
| Solute Carrier 26 family member A9 (Slc26a9) anion transporter in the inward-facing state | Mus musculus | Cryo-EM single particle analysis | 3.96 Å | 6RTC |
| SLC4 transporter Bor1p in an inward-facing conformation | Saccharomyces mikatae | X-ray diffraction | 5.9 Å | 5SV9 |
| OATP1B1 | Homo sapiens | Cryo-EM single particle analysis | 3.6 Å | 8PHW |
| Proton-coupled folate transporter at pH 6.0 bound to pemetrexed | Gallus gallus | Cryo-EM single particle analysis | 3.3 Å | 7BC7 |
| Concentrative nucleoside transporter CNT3 | Homo sapiens | Cryo-EM single particle analysis | 3.6 Å | 6KSW |
| b(0,+)AT1 | Homo sapiens | Cryo-EM single particle analysis | 3.4 Å | 6YV1 |
| Sulfate transporter AtSULTR4;1 | Arabidopsis thaliana | Cryo-EM single particle analysis | 2.75 Å | 7LHV |
| A bacterial dicarboxylate/sodium symporter | Vibrio cholerae | X-ray diffraction | 3.196 Å | 4F35 |
| SLC26A9 | Homo sapiens | Cryo-EM single particle analysis | 2.6 Å | 7CH1 |
| Peptide transporter PepT2 | Lama glama | Cryo-EM single particle analysis | 3.5 Å | 7NQK |
Table 1. Structural research of the solute carrier (SLC) transporter superfamily.
Structural analysis of the solute carrier (SLC) transporter superfamily is essential for revealing the regulatory mechanisms of multiple physiological processes. Structural research can help to understand the functional mechanism of SLC in disease formation, signaling pathways, and interactions with other proteins. It provides strategies for developing new attractive drug targets.
Creative Biostructure is a leading biotech company focused on protein structural biology. We provide SLC and various kinds of services related to protein structure research, such as X-ray crystallography, cryo-electron microscopy (cryo-EM), and nuclear magnetic resonance (NMR) services. Our professionals have extensive experience and cutting-edge equipment to provide comprehensive support for your research. If you are interested in protein structure analysis and related areas, please feel free to contact us for more details.
References
- Xie T, et al. Rational exploration of fold atlas for human solute carrier proteins. Structure. 2022. 30(9): 1321-1330. e5.
- Pizzagalli MD, et al. A guide to plasma membrane solute carrier proteins. FEBS J. 2021. 288(9): 2784-2835.
- Bai X, et al. Structural biology of solute carrier (SLC) membrane transport proteins. Mol Membr Biol. 2017. 34(1-2): 1-32.
- Ferrada E, Superti-Furga G. A structure and evolutionary-based classification of solute carriers. iScience. 2022. 25(10): 105096.
