Exosome Isolation by Density Gradient Centrifugation (DGC)
Exosomes transport various biomolecules, such as proteins, nucleic acids, and lipids, between cells. Recently, interest in exosomes has increased dramatically due to their great potential in the diagnosis and treatment of several cancers. The first step in research is to isolate exosomes from highly abundant free proteins and similarly sized lipoproteins. Research has found that density gradient centrifugation (DGC) is a useful and efficient method for exosome isolation.
What is DGC Technology?
The DGC technology is useful for many cell biology applications, including the isolation of organelles and the determination of the oligomeric state of proteins. It is used in combination with ultracentrifugation (UC) for the isolation of exosomes from biological fluids. It can be a good solution to the problem of co-precipitation due to the overlap of physical properties when isolating exosomes using UC.
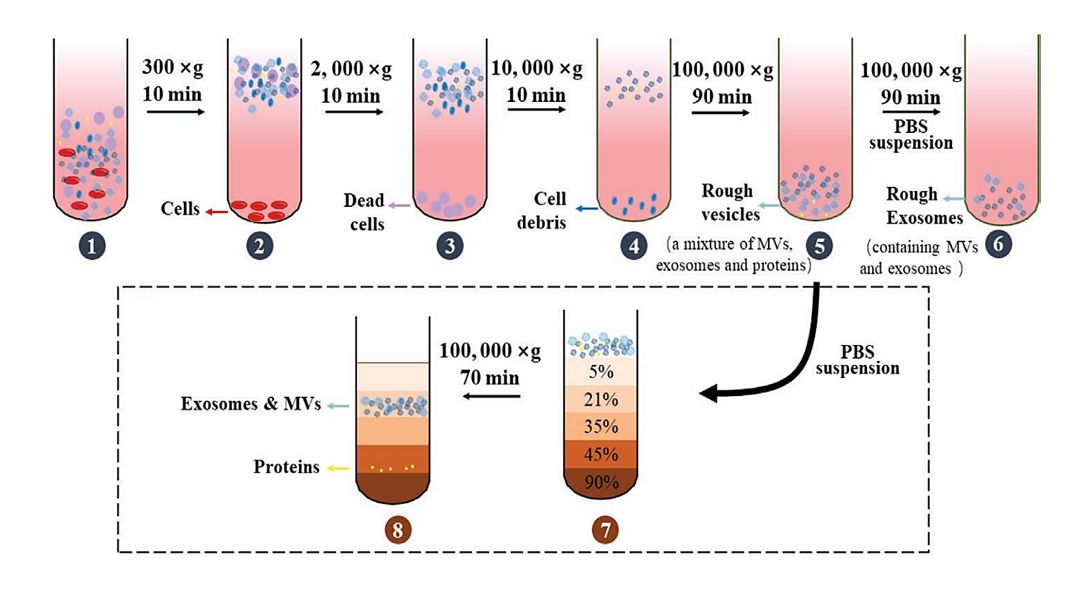 Figure 1. Diagram of density gradient centrifugation. (Xie Y, et al., 2022)
Figure 1. Diagram of density gradient centrifugation. (Xie Y, et al., 2022)
Creative Biostructure has been committed to the research and application of exosomes in the past decades, and we have rich experience in exosome preparation service, mastering DGC technology for efficient production. This method separates vesicles from particles of different densities and is capable of extracting low levels of exosomes.
How Does DGC Technology Work?
The DGC technology separates exosomes by adding solutions of different densities. The specific steps are as follows:

1. First, filtrate or centrifuge the samples to remove debris and large particles that may interfere with the separation of exosomes. This process also dilutes the samples so that they can pass through the concentration gradient for the required extended centrifugation time.
2. Layering the samples at the top of the density gradient and centrifuging them at high speed for several hours, depending on the type of biofluid, separates the different components of the samples.
3. After centrifugation, samples are collected from each density portion of the gradient and exosomes will be found in the denser portions. The collected exosomes can be used for downstream characterization and analysis.
Advances in DGC Technology Research
Researchers have proposed a one-step sucrose cushion buffer centrifugation (SUC) method in which body fluids or cell supernatants are added directly to a sucrose cushion, which is then collected and centrifuged to obtain exosomes after dilution with PBS. This method improves the yield and integrity of the resulting exosomes.
In recent years, the existing method has been improved by proposing buffered density gradient ultracentrifugation (C-DGUC), in which iodixanol buffer is used as a density gradient medium for concentrating exosomes. Iodixanol buffer can better preserve the physical and biological integrity of exosomes. In addition, since iodixanol is biologically inert and compatible, it does not need to be removed, thereby eliminating an additional step. C-DGUC greatly improves the yield and purity of sEVs and has been shown to allow the extraction of sEVs from plasma and urine, which holds great promise for clinical research and diagnostics.
Which Samples Can DGC Isolate Exosomes?
DGC is a widely used technique for isolating exosomes from a variety of biological samples, including populations of exosomes from cells, tissues, and biological fluids such as blood, urine, and cerebrospinal fluid. DGCs are particularly effective in separating exosomes based on their buoyant density. This versatility makes the DGC an invaluable tool for researchers studying exosome biology and utilizing exosomes for diagnostic, therapeutic, or biomarker discovery.
As a leader in the field of exosome research, Creative Biostructure offers clients a comprehensive range of high-quality exosome products that can be used directly for exosome characterization and functional analysis.
| Cat No. | Product Name | Source |
| Exo-HDBF-01 | HQExo™ Exosome-SDH-Alzheimer's plasma | Exosome derived from Single Donor Human Alzheimer's plasma |
| Exo-HDBF-02 | HQExo™ Exosome-SDH-Asthma plasma | Exosome derived from Single Donor Human Asthma plasma |
| Exo-HDBF-03 | HQExo™ Exosome-SDH-Atopic Dermatitis plasma | Exosome derived from Single Donor Human Atopic Dermatitis plasma |
| Exo-HDBF-04 | HQExo™ Exosome-SDH-Benign Breast Conditions plasma | Exosome derived from Single Donor Human Benign Breast Conditions plasma |
| Exo-HDBF-05 | HQExo™ Exosome-SDH-Benign Organ Tumor plasma | Exosome derived from Single Donor Human Benign Organ Tumor plasma |
| Exo-HDBF-06 | HQExo™ Exosome-SDH-Benign Prostatic Hyperplasia plasma | Exosome derived from Single Donor Human Benign Prostatic Hyperplasia plasma |
| Explore All Exosomes Isolated from Human Disease-state Body Fluids | ||
Why Choose DGC Technology?
- High Purity: DGC technology separates exosomes based on density. The centrifugation process effectively removes contaminants, resulting in purer exosome samples.
- Sample Source Flexibility: DGC technology can be used for a wide range of sample types and sizes and can handle both small and large batches.
- Scalability: DGC technology is a scalable methodology that allows exosomes to be isolated from small lab-scale samples to large industrial-scale batches.
- Gentle on Exosomes: DGC is a gentle isolation method that helps maintain exosome integrity and biological activity. Essential for downstream applications that rely on functional exosomes.
- Reliable and Proven: DGC has been widely used for exosome isolation for many years and is a proven technology with standardized protocols.
High purity and intact exosomes will contribute to downstream functional analyses. Creative Biostructure's skilled experts can help clients isolate and purify exosomes from various samples based on cutting-edge DGC technology. If you have any requirements for exosomes, please feel free to contact us for more information.
References
- Xie Y, et al. Effective Separation of Cancer-Derived Exosomes in Biological Samples for Liquid Biopsy: Classic Strategies and Innovative Development. Glob Chall. 2022. 6(9): 2100131..
- Jia Y, et al. Small extracellular vesicles isolation and separation: Current technologys, pending questions and clinical applications. Theranostics. 2022. 12(15): 6548-6575.
- Akbar A, et al. Methodologies to Isolate and Purify Clinical Grade Extracellular Vesicles for Medical Applications. Cells. 2022. 11(2): 186.