Nuclear Pore Complex Structure
The nuclear pore complex is a critical component of the nuclear envelope, a double membrane structure that surrounds the nucleus. NPCs facilitate the controlled exchange of molecules between the nucleus and the cytoplasm, including proteins, RNA, and other macromolecules. They play a critical role in gene expression, DNA repair, and cell cycle regulation. Dysfunction of NPCs has been implicated in several diseases, including cancer, neurodegenerative disorders, and viral infections. Studying the structure of NPCs provides critical insights into fundamental cellular processes such as cellular trafficking and gene regulation, disease mechanisms, and potential therapeutic interventions.
How Is Nuclear Pore Complex Organized?
The nuclear pore complex (NPC) is a large multiprotein assembly embedded in the nuclear envelope that spans both the inner and outer nuclear membranes. The NPC has structural features that include eightfold rotational symmetry, giving it a cylindrical shape with a central channel that facilitates the transport of molecules between the nucleus and the cytoplasm. It is composed of approximately 30 different proteins, known as nucleoporins (Nups), which aggregate to form various structural subcomplexes within the overall NPC architecture.
In detail, the core scaffold of the NPC is composed of nucleoporins that assemble into concentric rings, including the cytoplasmic ring, the nuclear ring, and the central channel. The cytoplasmic and nuclear rings project into the cytoplasm and nucleus, respectively, and are involved in docking of transport receptors and interactions with other cellular components. These rings are interconnected by spoke-like structures that serve to stabilize the entire NPC complex and help maintain its structural integrity.
Inside the central channel of the NPC, a network of intrinsically disordered nucleoporins known as FG-repeat proteins (rich in phenylalanine-glycine repeats) forms a selective barrier. This barrier allows the passive diffusion of small molecules while regulating the active transport of larger cargoes such as proteins and RNA through interactions with nuclear transport receptors such as importins and exportins.
Extending from the NPC into both the cytoplasm and nucleoplasm are long, flexible filaments known as the cytoplasmic filaments and the nuclear basket, respectively. The cytoplasmic filaments play a critical role in the initial capture and docking of transport complexes, while the nuclear basket is involved in RNA processing and export, chromatin organization, and gene regulation.
At the base of the NPC, where it anchors to the nuclear envelope, nucleoporins are responsible for forming a stable connection between the NPC and the surrounding membrane, ensuring that the NPC remains properly positioned and functional. This connection is facilitated by membrane-bending proteins that help to accommodate the curvature of the nuclear envelope around the NPC.
Overall, the NPC is a highly modular and dynamic structure, capable of undergoing conformational changes to adapt to different cellular states, such as during cell division or in response to signaling events. Its complexity and precision are vital for maintaining cellular homeostasis, regulating gene expression, and ensuring the accurate transmission of genetic information across generations.
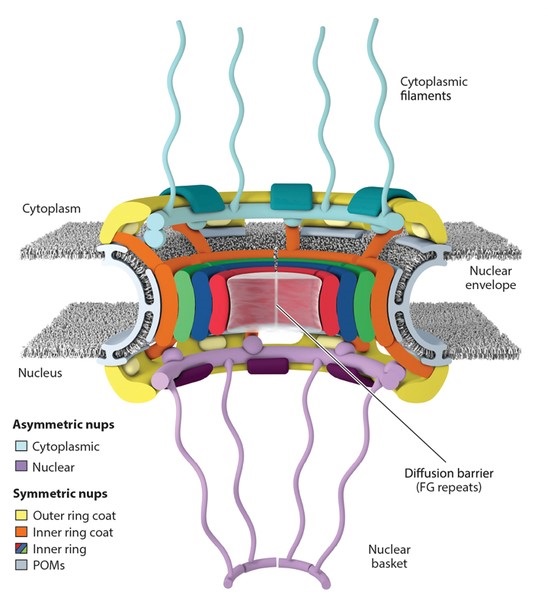 Figure 1. Schematic representation of the NPC architecture. A cutaway view depicting half of an NPC is shown (Lin and Hoelz, 2019).
Figure 1. Schematic representation of the NPC architecture. A cutaway view depicting half of an NPC is shown (Lin and Hoelz, 2019).
Components of Nuclear Pore Complex: Nucleoporins
As a family of proteins that make up the NPC, nucleoporins are diverse in structure and function, with around 30 different types identified in humans. These proteins can be classified into several classes based on their roles within the NPC:
- Structural Nucleoporins: These provide the scaffolding for the NPC, forming the basic framework that holds the complex together. They include proteins that form the ring structures and other key architectural elements.
- FG-Nucleoporins: Named for their repeating phenylalanine-glycine (FG) motifs, these nucleoporins line the central channel of the NPC and interact with transport receptors to facilitate the selective passage of molecules. They play a critical role in the active transport of proteins and RNA.
- Peripheral Nucleoporins: These nucleoporins are located on the cytoplasmic and nuclear sides of the NPC and are involved in anchoring and positioning the NPC within the nuclear envelope.
Nucleoporins are essential for maintaining cellular function and integrity, and defects in these proteins can lead to a variety of diseases, including certain cancers and neurological disorders. Their study is critical to understanding the mechanisms of nuclear transport and the overall regulation of gene expression.
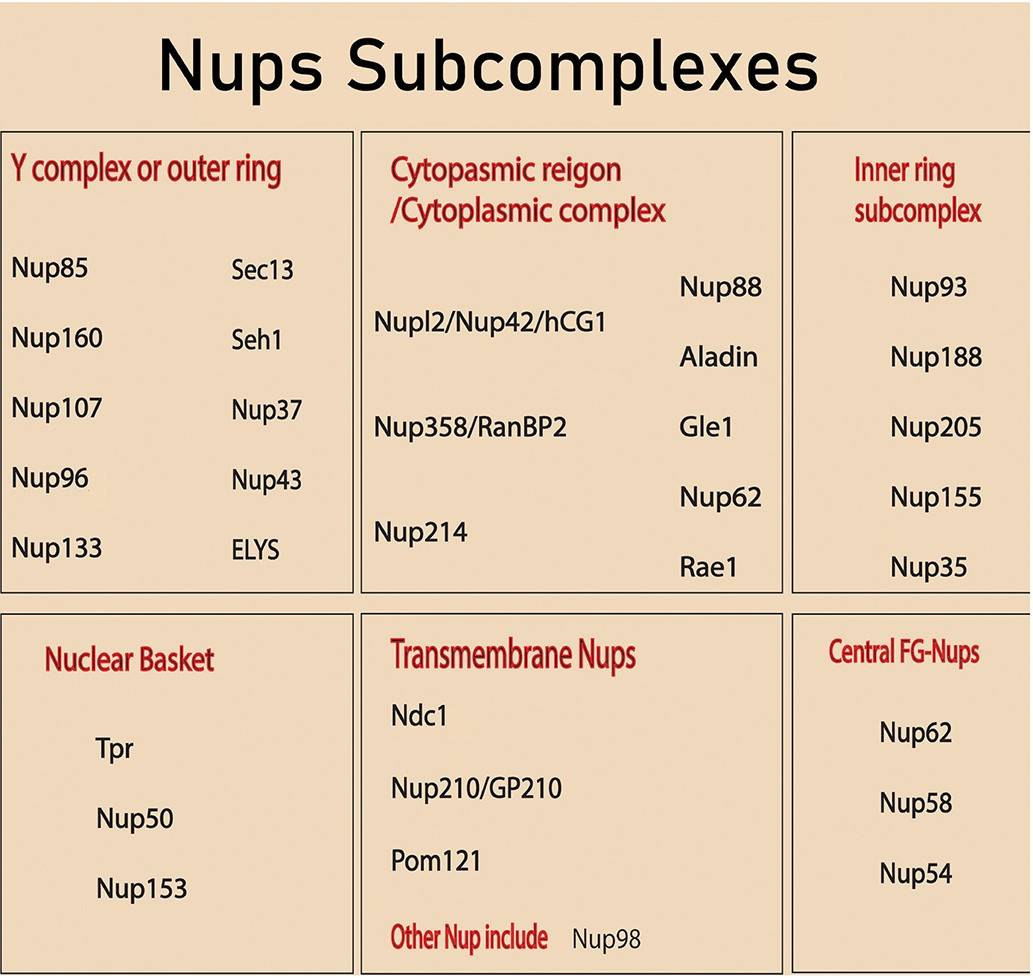 Figure 2. NPC building Nups subcomplexes. (Khan et al., 2020).
Figure 2. NPC building Nups subcomplexes. (Khan et al., 2020).
Dynamics of Nuclear Pore Complex
The structure and function of NPCs are highly dynamic and subject to regulation throughout the cell cycle and in response to various cellular signals.
Cell Cycle Regulation: During mitosis, NPCs undergo extensive remodeling to facilitate chromosome segregation. The nuclear envelope disassembles, and NPC components are either removed or modified to allow the formation of mitotic spindles. Following mitosis, NPCs are reassembled in the daughter nuclei, a process that involves the reorganization of nucleoporins and the reformation of the nuclear envelope.
Transport Dynamics: The central channel of the NPC is not static, but rather exhibits dynamic conformational changes to regulate the passage of molecules. Transport through the NPC is mediated by nuclear transport receptors, such as importins and exportins, which interact with specific nucleoporins to facilitate the movement of cargo through the pore.
Response to Cellular Signals: NPCs respond to various cellular signals and environmental conditions. For example, under conditions of stress or DNA damage, the function and composition of NPCs can be altered to regulate the transport of stress-related factors and repair proteins.
Nuclear Pore Complex Lifecycle
The lifecycle of the nuclear pore complex involves several stages, including assembly, function, and disassembly.
Assembly: NPC assembly begins in late telophase and early G1 of the cell cycle. Newly synthesized nucleoporins are recruited to the nuclear envelope where they undergo complex interactions to form the NPC structure. This process requires the coordination of multiple assembly factors and chaperones.
Function: Once assembled, NPCs facilitate the exchange of molecules between the nucleus and the cytoplasm. They regulate the transport of proteins, RNA, and other macromolecules, ensuring that only properly processed and correctly sized molecules are transported. NPCs also play a role in maintaining the shape and organization of the nucleus.
Disassembly and Recycling: During mitosis, NPCs disassemble to allow for chromosome segregation. Components of the NPC are either recycled for reassembly or degraded. After mitosis, NPCs are reassembled in the daughter nuclei, a process that involves the reconstitution of the central channel and peripheral rings.
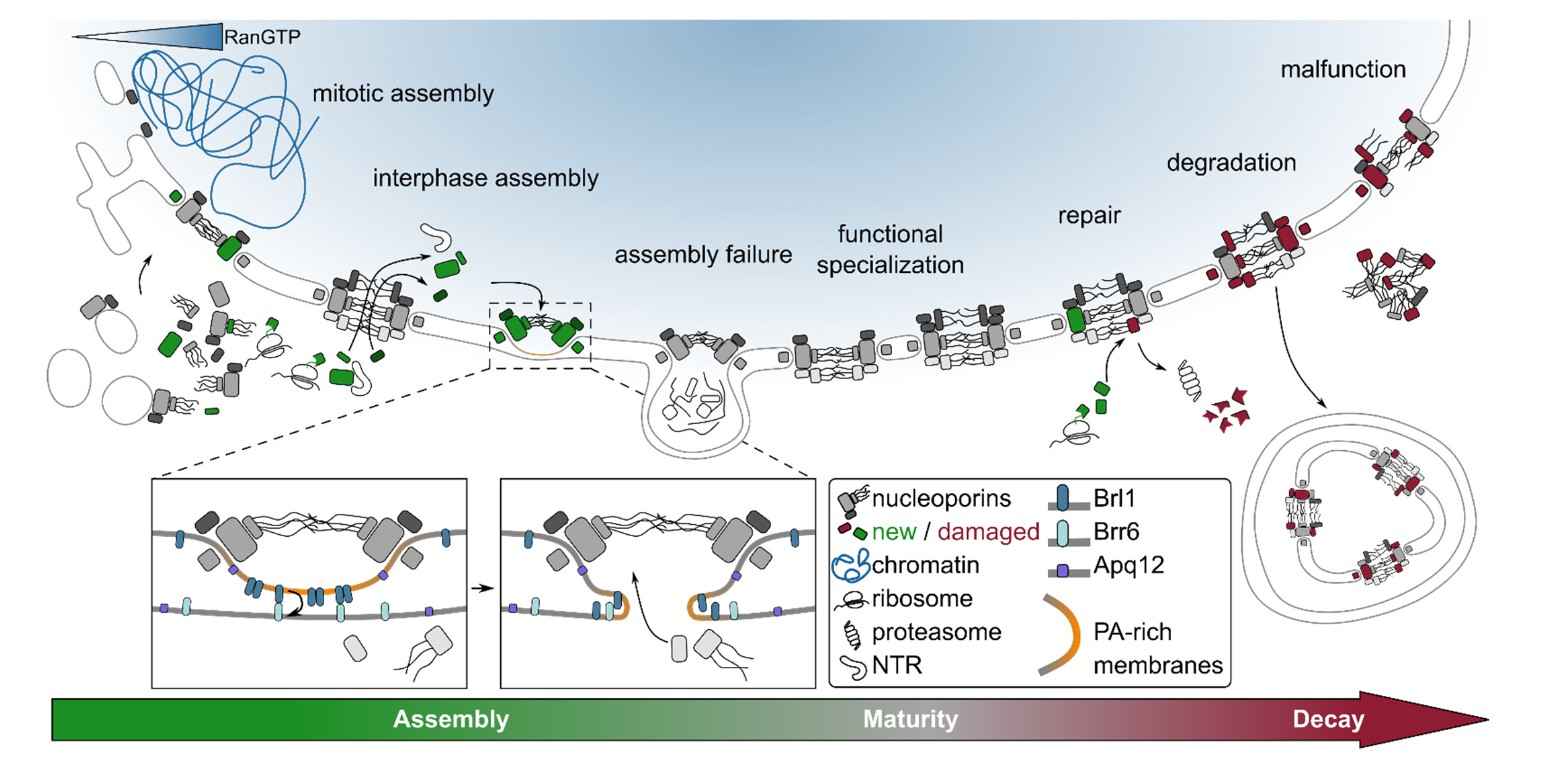 Figure 3. The lifecycle of the NPC. A range of proteins are involved in this process including nucleoporins, Brl1, Brr6, Apq12 (Dultz et al., 2022).
Figure 3. The lifecycle of the NPC. A range of proteins are involved in this process including nucleoporins, Brl1, Brr6, Apq12 (Dultz et al., 2022).
Methods for Analyzing Nuclear Pore Complex Structure
Several advanced techniques are employed to study the structure and dynamics of nuclear pore complexes, each providing unique insights into their organization and function.
| Techniques | Description |
| Electron Microscopy (EM) | EM, in particularly cryo-electron tomography, allows for high-resolution imaging of NPCs in their native cellular environment. This technique provides detailed structural information about the overall organization of NPCs and their components. |
| X-ray Crystallography | X-ray crystallography is used to determine the atomic resolution structure of individual nucleoporins and other NPC components. This technique provides insight into the precise arrangement of proteins within the NPC. |
| Nuclear Magnetic Resonance (NMR) Spectroscopy | NMR spectroscopy can be used to study the structure and dynamics of nucleoporins and other proteins involved in NPC function. This technique provides information about protein conformations and interactions at the molecular level. |
| Fluorescence Spectroscopy | Fluorescence microscopy, including techniques such as fluorescence recovery after photobleaching (FRAP) and fluorescence resonance energy transfer (FRET), allows for the study of NPC dynamics and protein interactions in living cells. These techniques provide real-time information on the mobility and behavior of NPC components. |
| Mass Spectrometry | Mass spectrometry is used to analyze the protein composition of NPCs and to identify post-translational modifications. This technique helps to understand the composition and functional roles of different nucleoporins and associated proteins. |
| Chromatin Immunoprecipitation (ChIP) | ChIP combined with high-throughput sequencing (ChIP-seq) can be used to study the interactions between NPCs and chromatin. This approach provides insight into how NPCs affect gene expression and chromatin organization. |
The nuclear pore complex is a highly organized and dynamic structure that plays a critical role in regulating nucleocytoplasmic transport and maintaining nuclear architecture. Its intricate structure, consisting of multiple nucleoporins and dynamic components, is essential for its function in facilitating the exchange of molecules between the nucleus and the cytoplasm.
At Creative Biostructure, we offer advanced cryo-EM solutions for subcellular organelles to investigate the nuclear pore complex, supporting your research into cellular organization and the mechanisms of related diseases. Contact to our experts for detailed analysis and innovative solutions tailored to your needs.
References
- D'Angelo, M. A., & Hetzer, M. W. (2008). Structure, dynamics and function of nuclear pore complexes. Trends in Cell Biology, 18(10), 456–466.
- Dultz, E., Wojtynek, M., Medalia, O., & Onischenko, E. (2022). The nuclear pore complex: Birth, life, and death of a cellular behemoth. Cells, 11(9), 1456.
- Khan, A. U., Qu, R., Ouyang, J., & Dai, J. (2020). Role of nucleoporins and transport receptors in cell differentiation. Frontiers in Physiology, 11.
- Lin, D. H., & Hoelz, A. (2019). The structure of the nuclear pore complex (An update). Annual Review of Biochemistry, 88, 725–783.
- Strambio-De-Castillia, C., Niepel, M., & Rout, M. P. (2010). The nuclear pore complex: Bridging nuclear transport and gene regulation. Nature Reviews Molecular Cell Biology, 11(7), 490–501.