Mempro™ Bacteriophage Qβ for Virus-like Particles (VLPs)
Creative Biostructure has established an unparalleled virus-like particles (VLPs) platform with years of experience, Scientists from Creative Biostructure can provide high quality of tailored Mempro™ VLPs derived from bacteriophage Qβ.
VLPs are resemble to viruses without any viral genetic materials so they can’t replicate in the hosts. Generally, VLPs are able to self-assemble by expressing viral structural proteins, capsid or envelope for example. Several cell culture systems are used to generate VLPs: mammalian cell lines, yeast and plant cells, insect cell lines and bacteria. Due to the special features of VLPs, they are crucial in the progress of vaccines, virus research, therapy and lipoparticle technology.
Bacteriophage Qβ is one of the members of leviviridae family, and it has a 4.2 kb sense strand RNA genome encircled by the icosahedral capsid which is composed of 180 coat protein subunits. The virus is one of the diminutive RNA bacteriophages infecting Escherichia coli. There are four groups (I, II, III and IV) of bacteriophages and bacteriophage Qβ is regarded as group III due to its molecular biology.
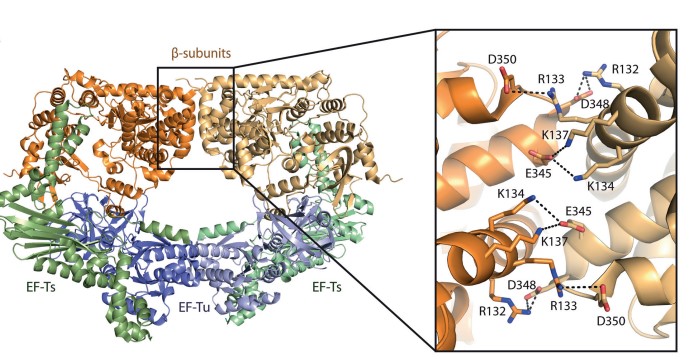
Figure 1. Structure of the dimer of the Qβ replicase core complex.( Nucleic acids research 2015)
- Structure of Bacteriophage Qβ
The crystal structure of bacteriophage Qβ particle indicated that the capsid protein postulates three disparate conformations and generates two unlike dimmer types with the same topology as those present in MS2. Duo to the disulfide bonds in covalent pentamers and hexamers, coat protein subunits of bacteriophage Qβ are connected together. Generally, there are 132 residues in the coat protein subunits, however the subunits three to five added an additional 196 residues by inhibition of the termination of translation.
- VLPs of Bacteriophage Qβ
During the native infection, bacteriophage Qβ virion reveals three to five copies of a great domain extension at the C-terminus (nearly 200 amino acids), named as the A1 protein which is used to recognize the Escherichia coli host. In addition, bacteriophage Qβ coat protein forms unified virus-like particles when expressed recombinantly in all kinds of organisms. Researches have produced mutant recombinant virus-like particles that incorporated C-terminal stretches using fragments of the A1 sequence up to the full-length domain.
With years of experience in VLPs and bacteriophage Qβ research, Creative Biostructure can perform world-leading services of VLPs design & production and VLPs characterization of bacteriophage Qβ. Please feel free to contact us for a detailed quote.
References:
Golmohammadi R, Fridborg K, Bundule M, et al. The crystal structure of bacteriophage Qβ at 3.5 Å resolution[J]. Structure, 1996, 4(5): 543-554.
Gytz H, Mohr D, Seweryn P, et al. Structural basis for RNA-genome recognition during bacteriophage Qβ replication[J]. Nucleic acids research, 2015, 43(22): 10893-10906.
Rumnieks J, Tars K. Crystal structure of the bacteriophage Qβ coat protein in complex with the RNA operator of the replicase gene[J]. Journal of molecular biology, 2014, 426(5): 1039-1049.