In Situ Administration Exosomes
Exosome in situ administration (ISA) refers to the direct targeted delivery of exosomes to specific tissues or organs to achieve a localized therapeutic effect. This approach aims to maximize the therapeutic potential of exosomes by targeting them at specific sites. ISA reduces off-target effects, significantly increases the local concentration of exosomes, and improves their bioavailability. At the same time, the method reduces the exposure of exosomes to non-target tissues and may reduce the risk of adverse side effects.
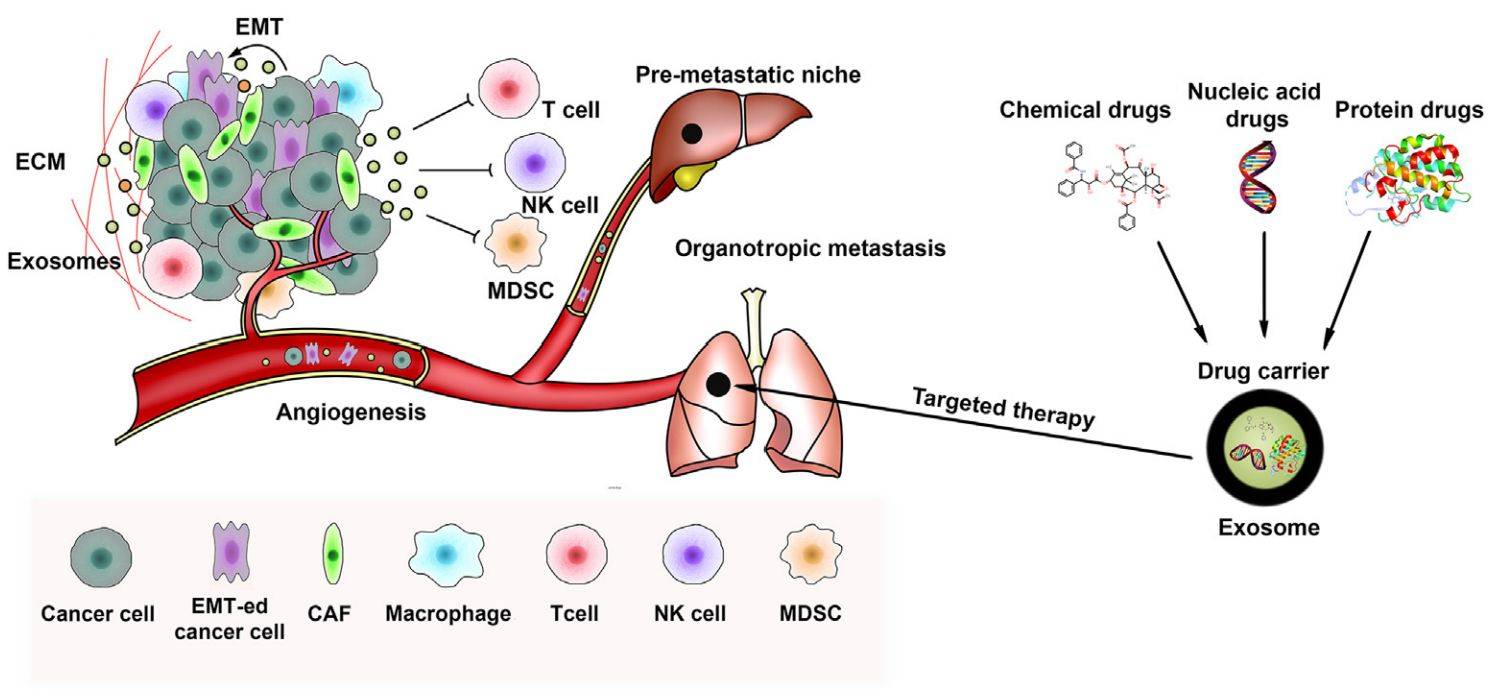 Figure 1. Schematic of exosomes in tumor metastasis and targeted therapy. (Liu J, et al., 2021)
Figure 1. Schematic of exosomes in tumor metastasis and targeted therapy. (Liu J, et al., 2021)
Overview of Exosome ISA
Several strategies for exosome in situ delivery include direct injection, implantation, or incorporation into biomaterials or scaffolds. These approaches can be customized based on the target site's specific application and anatomical location. For example, in regenerative medicine, exosomes have been ISA to promote tissue repair and regeneration, such as in the case of cardiac, musculoskeletal, or neurological disorders.
Preclinical studies have demonstrated the effectiveness of in situ exosome administration in a variety of animal models. Researchers have reported improved functional outcomes, enhanced tissue healing, and reduced inflammation following topical exosome administration. In addition, the use of engineered or modified exosomes expands the possibilities for targeted therapies in which exosome cargo or surface properties can be tailored to treat specific diseases.
Application Fields of Exosome ISA
Regenerative Medicine - Exosomes can be ISA to promote tissue regeneration and repair, for example in orthopedic injuries, skin wounds, and cardiovascular disease.
Oncology - Exosomes can be designed to deliver anticancer agents directly to solid tumors, thereby improving drug delivery and reducing systemic toxicity.
Neurological Disorders - Exosomal ISAs can facilitate the targeted delivery of neuroprotective factors to the central nervous system for the treatment of conditions such as spinal cord injury, stroke, and neurodegenerative diseases.
Inflammatory/Immune Diseases - Exosomes can be used to modulate the immune system in situ and reduce autoimmune diseases and chronic inflammation.
Cases
- In Situ Sprayed Exosome Crosslinking Gel for Postoperative Glioblastoma Immunotherapy
A key challenge in postoperative glioblastoma immunotherapy is to ensure a strong and sustained T-cell response. Researchers have developed an in situ sprayed exosome cross-linking gel that directly activates tumor-infiltrating T cells, thereby preventing glioma recurrence. This gel is produced by a bioorthogonal reaction between an azide-modified chimeric exosome and an alkyne-modified alginate polymer. The gel with chimeric exosomes as cross-linking sites avoids rapid clearance by the immune system, thus prolonging the durability of anti-tumor T-cell immunity. The findings provide a promising strategy for postoperative glioma immunotherapy.
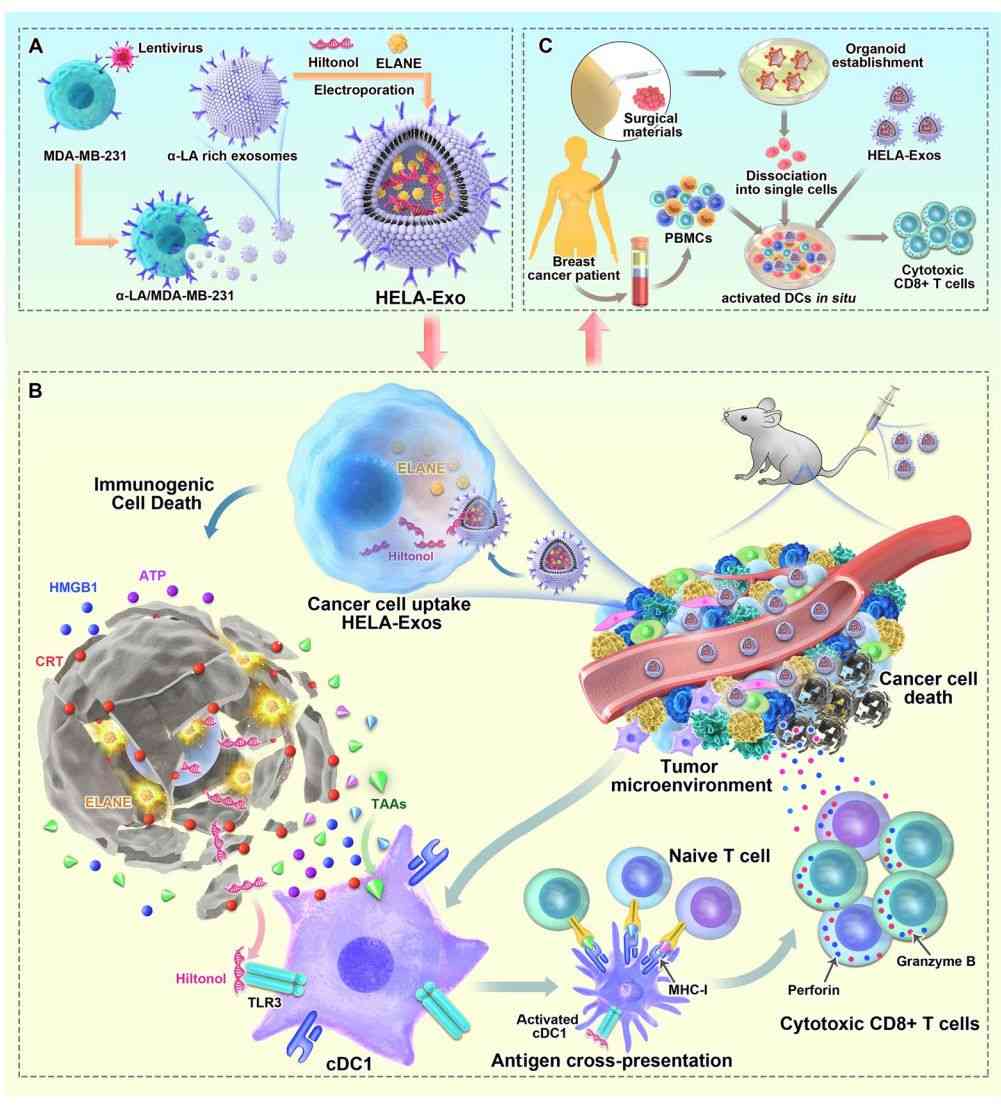 Figure 2. In situ sprayed exosome cross-linked gel for postoperative glioblastoma immunotherapy. (Huang L, et al., 2022)
Figure 2. In situ sprayed exosome cross-linked gel for postoperative glioblastoma immunotherapy. (Huang L, et al., 2022)
- Exosomes as In Situ Dendritic Cell-Initiated Vaccines Enhance Antitumor Immunity
Dendritic cells (DCs) are central to initiating and regulating the tumor microenvironment. Several vaccines targeting DCs have been developed to improve cancer immunotherapy. Researchers loaded the immunogenic cell death (ICD) inducers ELANE and Hiltonol (TLR3 agonist) into α-lactalbumin (α-LA)-engineered breast cancer-derived exosomes to form an in situ DC vaccine (HELA-Exos). It has been found that after HELA-Exo induces ICD in cancer cells, sufficient exposure to tumor antigens and Hiltonol activates conventional dendritic cell type I (cDC1) in situ and triggers a tumor-reactive CD8+ T-cell response, resulting in potent tumor suppression.
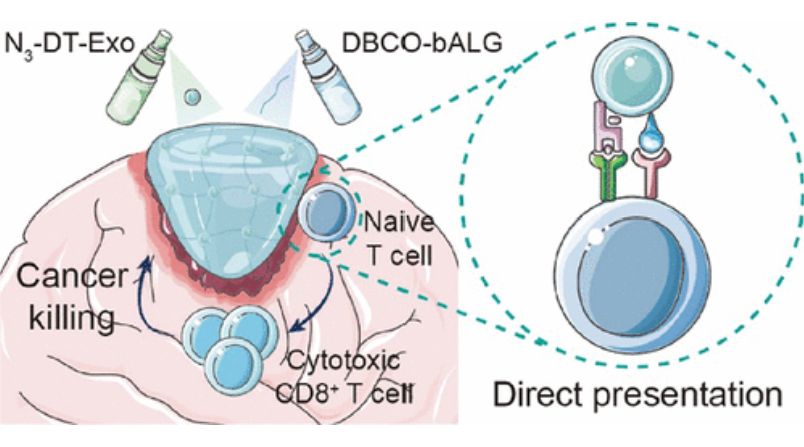 Figure 3. Schematic of HELA-Exos as an in situ DC-initiated vaccine for breast cancer. (Bao P, et al., 2024)
Figure 3. Schematic of HELA-Exos as an in situ DC-initiated vaccine for breast cancer. (Bao P, et al., 2024)
Benefits of Exosome ISA
- Targeted Delivery - ISA can precisely localize exosomes to the site of interest to maximize therapeutic efficacy and minimize off-target effects.
- Improved Bioavailability - Direct administration to the target site significantly increases the local concentration of exosomes, enhancing their bioavailability and therapeutic efficacy.
- Reduced Systemic Exposure - By avoiding systemic circulation, ISA can reduce exosome exposure to non-target tissues, potentially reducing the risk of adverse side effects.
- Enhanced Therapeutic Effectiveness - Targeted delivery and increased bioavailability of exosomes at the site of action can lead to better therapeutic effects in the context of localized inflammation compared to systemic administration.
Progress and Challenges in Exosome ISA Research
Research Progress
- Advances in exosome engineering and surface modification have enabled the development of exosomes with enhanced targeting capabilities for in situ delivery.
- Preclinical studies have demonstrated the feasibility and efficacy of exosomal ISAs in various disease models such as wound healing, cartilage regeneration, and brain cancer.
- Clinical trials are exploring the safety and efficacy of exosome ISAs in human patients and have yielded encouraging early results.
Challenges
- Optimize ISA delivery methods and formulations to ensure exosome stability and integrity.
- Develop reliable and scalable methods to isolate and purify exosomes suitable for in situ applications.
- Ensure the safety and efficacy of exosome-based ISA therapies.
Today We Offer
Creative Biostructure is a leading provider of exosome solutions, helping clients explore the exciting applications of exosome ISA. As experts in exosome research, our team specializes in the isolation, characterization, and analysis of exosomes for targeted therapies. Whether it's enhancing tissue regeneration, enabling localized drug delivery, or advancing cancer immunotherapy, our exosome technology enables clients to push the limits of regenerative medicine and targeted therapy. Please feel free to contact us for more information.
| Cat No. | Product Name | Source |
| Exo-CH06 | HQExo™ Exosome-MCF-7 | Exosome derived from human breast cancer, noninvasive cell line (MCF-7 cell line) |
| Exo-CH07 | HQExo™ Exosome-MDA-MB-231 | Exosome derived from human breast cancer, aggressive/invasive/metastatic cell line (MDA-MB-231 cell line) |
| Exo-CH10 | HQExo™ Exosome-HCT116 | Exosome derived from human colorectal carcinoma cell line (HCT116 cell line) |
| Exo-CH04 | HQExo™ Exosome-H196 | Exosome derived from Human small cell lung cancer cell line (H196 cell line) |
| Exo-CH09 | HQExo™ Exosome-DAUD1 | Exosome derived from human burkitt lymphoma cell line (DAUD1 cell line) |
| Exo-CH16 | HQExo™ Exosome-COLO1 | Exosome derived from human colon carcinoma (COLO1 cell line) |
| Exo-SC01 | HQExo™ Exosome-PCS-500-011 | Exosome derived from human pre-adipose derived mesenchymal stem cell (PCS-500-011) |
| Exo-SC03 | HQExo™ Exosome-hTERT | Exosome derived from hTERT-immortalized Mesenchymal Stem Cell |
| Exo-SC04 | HQExo™ Exosome-MSC | Exosome derived from Xeno-Free Human Mesenchymal Stem/Stromal Cells and Media |
| Exo-IC03 | HQExo™ Exosome-Jurkat E6-1 | Exosome derived from human T lymphocyte cell line (Jurkat E6-1) |
| Exo-IC04 | HQExo™ Exosome-RAWS 264.7 | Exosome derived from mouse macrophage cell line (RAWS 264.7) |
| Exo-IC05 | HQExo™ Exosome-JAWSII | Exosome derived from mouse bone marrow immature dendritic cell line (JAWSII) |
| Exo-IC06 | HQExo™ Exosome-NK | Exosome derived from human NK cell line |
| Explore All Exosome Products | ||
References
- Liu J, et al. The biology, function, and applications of exosomes in cancer. Acta Pharm Sin B. 2021. 11(9): 2783-2797.
- Huang L, et al. Engineered exosomes as an in situ DC-primed vaccine to boost antitumor immunity in breast cancer. Mol Cancer. 2022. 21(1): 45.
- Bao P, et al. In Situ Sprayed Exosome-Cross-Linked Gel as Artificial Lymph Nodes for Postoperative Glioblastoma Immunotherapy. ACS Nano. 2024. 18(20): 13266-13276.