Exosomes Loading Drugs by Electroporation
The clinical application of chemotherapeutic agents is limited by several factors, including low cellular uptake, short circulation time, and severe adverse effects. Exosomes, which are cell-derived lipid bilayer nanoparticles involved in cell-to-cell communication, are recognized as drug-carrying platforms with the potential to overcome these barriers. They can transport bioactive cargoes throughout the body, across biological barriers, and target a variety of tissues. Several small molecule drugs have been successfully incorporated into exosomes, allowing them to be efficiently transported to tumor tissues, improving therapeutic efficacy and reducing adverse effects. However, cargo loading is often insufficient and selecting an appropriate loading method is a prerequisite for successfully utilizing the platform. In recent years, electroporation has been widely used for exosome drug loading. It applies an external electric field outside the exosome membrane and uses short high-voltage pulses to overcome the membrane barrier to load the drug into the exosome.
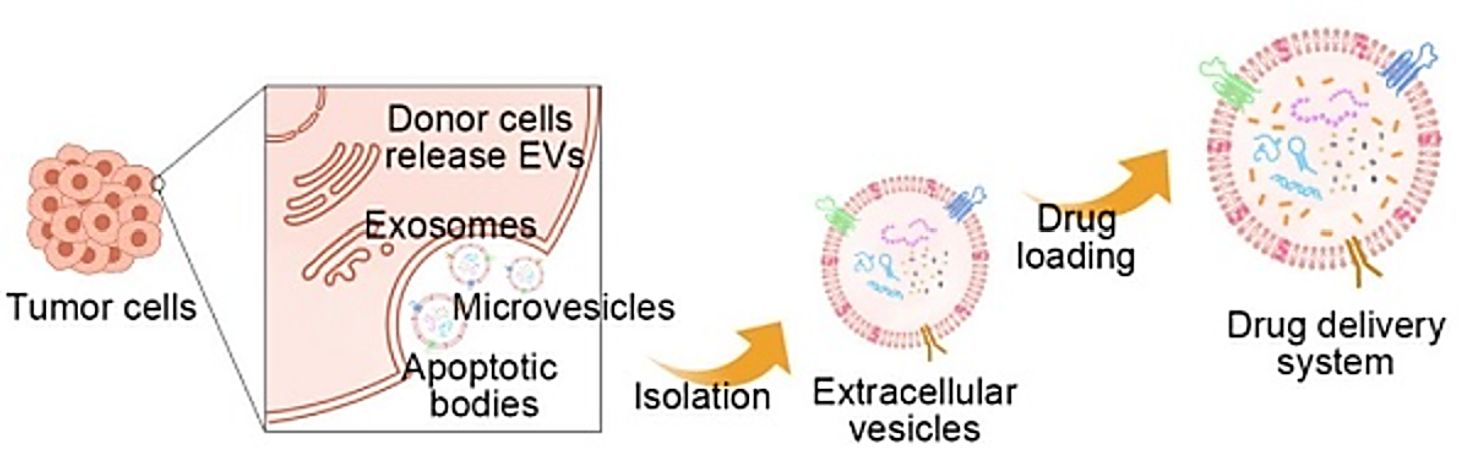 Figure 1. Tumor-derived extracellular vesicles as drug carriers in cancer therapy. (Xu Y, et al., 2022)
Figure 1. Tumor-derived extracellular vesicles as drug carriers in cancer therapy. (Xu Y, et al., 2022)
What is Electroporation?
- Overview of Electroporation
Electroporation, also known as electrotransfection, is the transient enhancement of the permeability of the exosome membrane through the action of a high-intensity electric field, thereby absorbing exogenous molecules from the surrounding medium. This technique allows the introduction of small molecules, nucleic acids, and protein drugs into exosomes. Electroporation creates small holes in the exosome membrane by applying an electric field to the exosome suspended in a conductive solution. The electric current disrupts the phospholipid bilayer of the exosome, resulting in the formation of temporary pores through which the drug can subsequently diffuse into the interior of the exosome. The integrity of the exosome membrane is restored at the end of the drug-loading process. This method is widely used to load relatively large nucleotides of siRNA or miRNA into exosomes. Research has shown that electroporation can load siRNA better than chemical transfection.
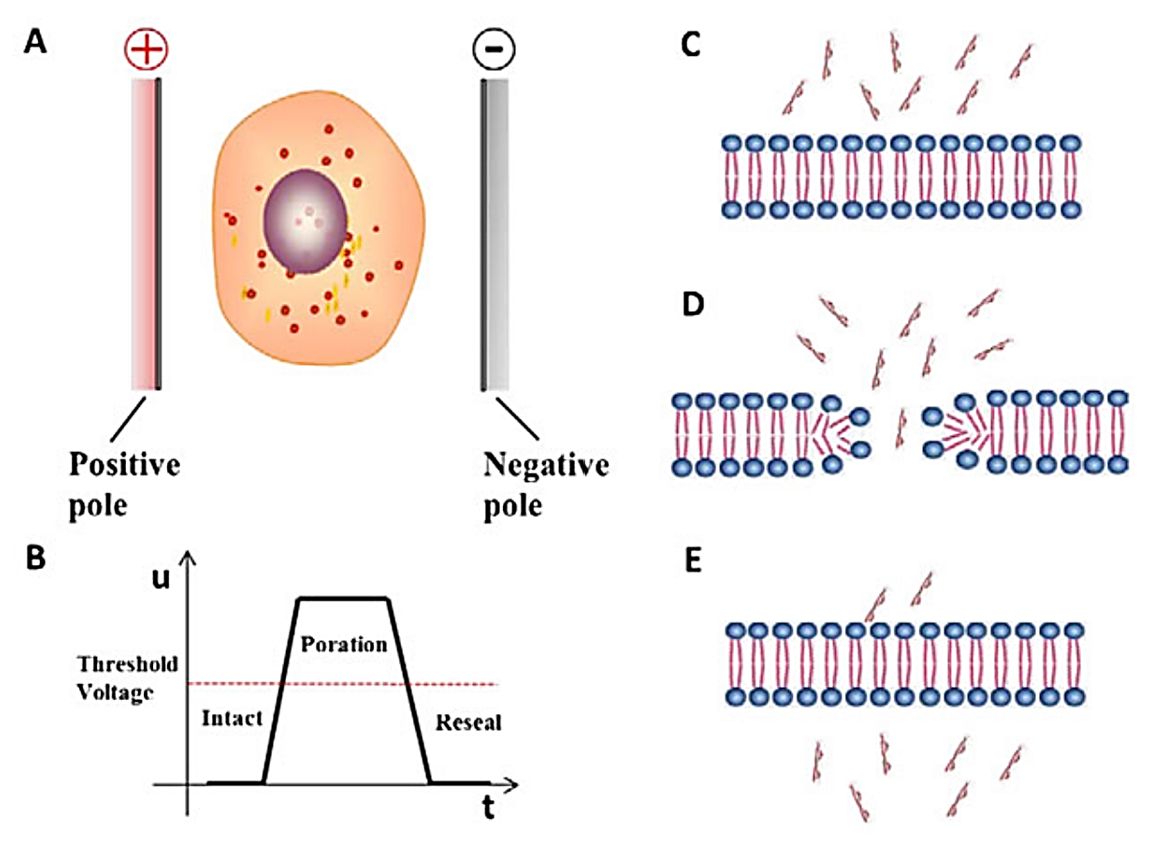 Figure 2. Schematic diagram of the principle of electroporation. (Du X, et al., 2018)
Figure 2. Schematic diagram of the principle of electroporation. (Du X, et al., 2018)
- Application Scope of Electroporation
Electroporation can be used for both small-molecule drugs and large-molecule substances (especially hydrophilic molecules) loading, in addition, electroporation is also considered as the preferred way to load nucleic acid molecules into exosomes. The voltage (140-700 V) and exosome concentration used for electroporation vary widely in different research, and since electroporation causes some damage to exosomes, it is often necessary to increase the amount of exosome input. Usually, a voltage of 140-200V and an exosome protein concentration of 250-1000g/mL are ideal conditions for loading RNA molecules (mainly miRNA/siRNA). For larger nucleic acid molecules, such as dsDNA, electroporation can also be used for loading, but the loading efficiency is affected by the molecular weight, and loading over 750 bp is not effective.
- Optimization of Electroporation
Factors such as pulse intensity, buffer medium, drug type, and input volume are considered to affect the efficiency of electroporation. When choosing to use electroporation for exosome drug loading, the condition settings need to be optimized. Since a smaller radius is associated with increased membrane stability, higher field strength is appropriately applied to achieve good membrane permeability when processing exosomes. Essential parameters for efficient electroporation include sample composition. To maximize the material efficiency of the subsequent steps, the relative concentrations of exosomes and drugs as well as the choice of buffer also need to be optimized.
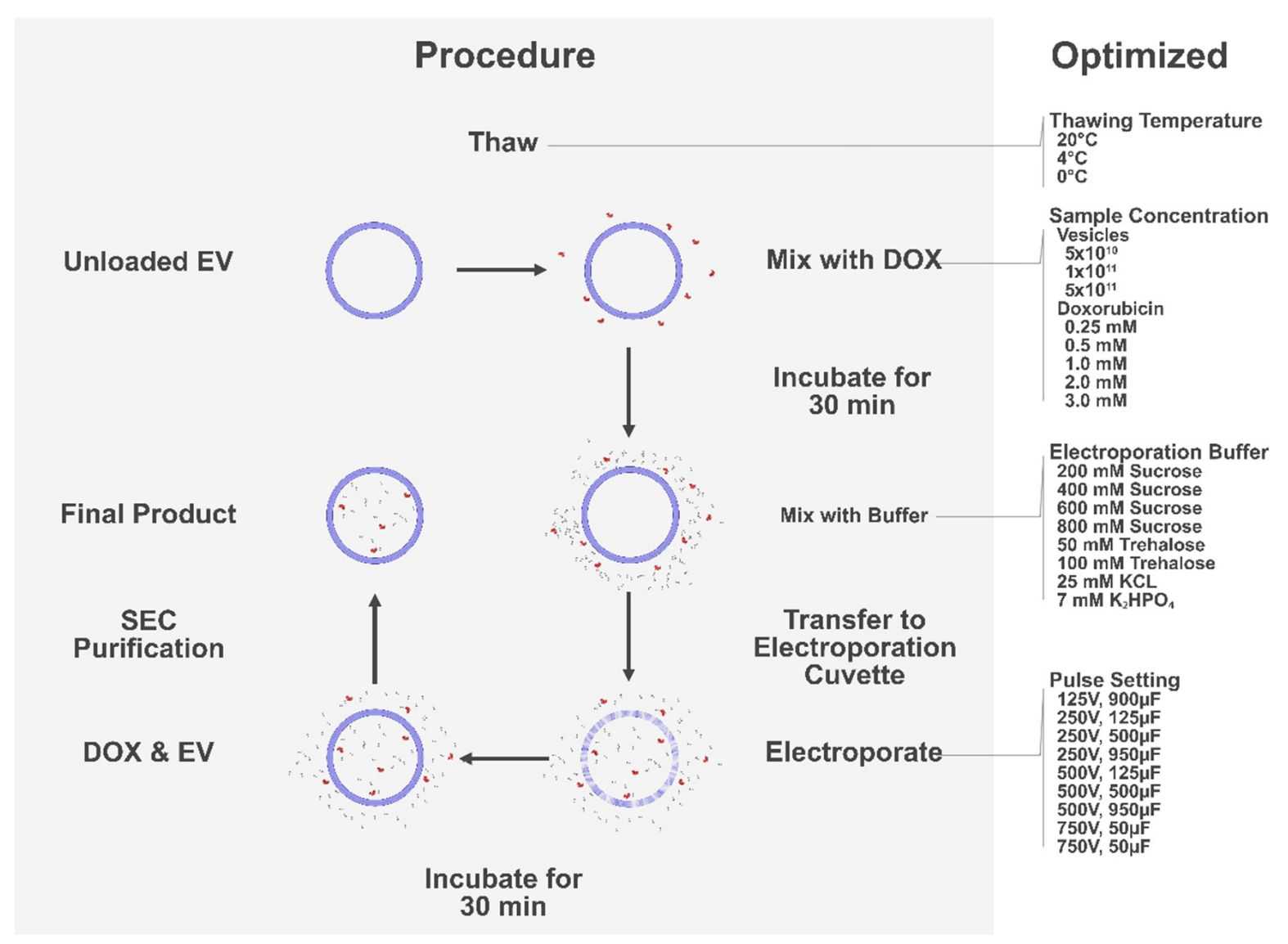 Figure 3. Schematic of the optimization process of electroporation. (Lennaárd AJ, et al., 2021)
Figure 3. Schematic of the optimization process of electroporation. (Lennaárd AJ, et al., 2021)
Procedure for Exosome Drug Loading by Electroporation
Sample preparation
- Collect and culture exosome samples of interest.
- Isolate the exosomes by ultracentrifugation centrifugation and resuspend them in the appropriate electroporation buffer.
- Prepare a solution of the target drug molecule.
Electroporate the exosomes
- Transfer the exosome-drug mixture to an electroporation cuvette.
- Place the cuvette in an electroporation device called an electroporator.
- Activate the electroporation device and apply an electrical pulse to the exosome.
Recover the exosomes
- Immediately after the end of the electroporation pulse, remove the cuvette from the electroporation device.
- Recover briefly at room temperature or on ice to reseal the exosome membrane.
Culture and harvest transfected exosomes
- After the recovery period, incubate exosomes under appropriate culture conditions according to experimental requirements.
Research Advances in Exosome Drug Delivery by Electroporation
CRISPR-Cas9 gene editing has emerged as a powerful therapeutic technology, but the lack of safe and effective targeted delivery systems has limited its clinical application. Delivery of Cas9 ribonucleoprotein (RNP) offers competitive advantages, however, the large size of RNP exceeds the loading capacity of currently available delivery vectors. Therefore, researchers have developed an innovative genome editing delivery system called exosomeRNP, in which Cas9 RNP is loaded into exosomes isolated from hepatic stellate cells by electroporation. The results show that exosomeRNP displays strong therapeutic potential in acute liver injury, chronic liver fibrosis, and hepatocellular carcinoma.
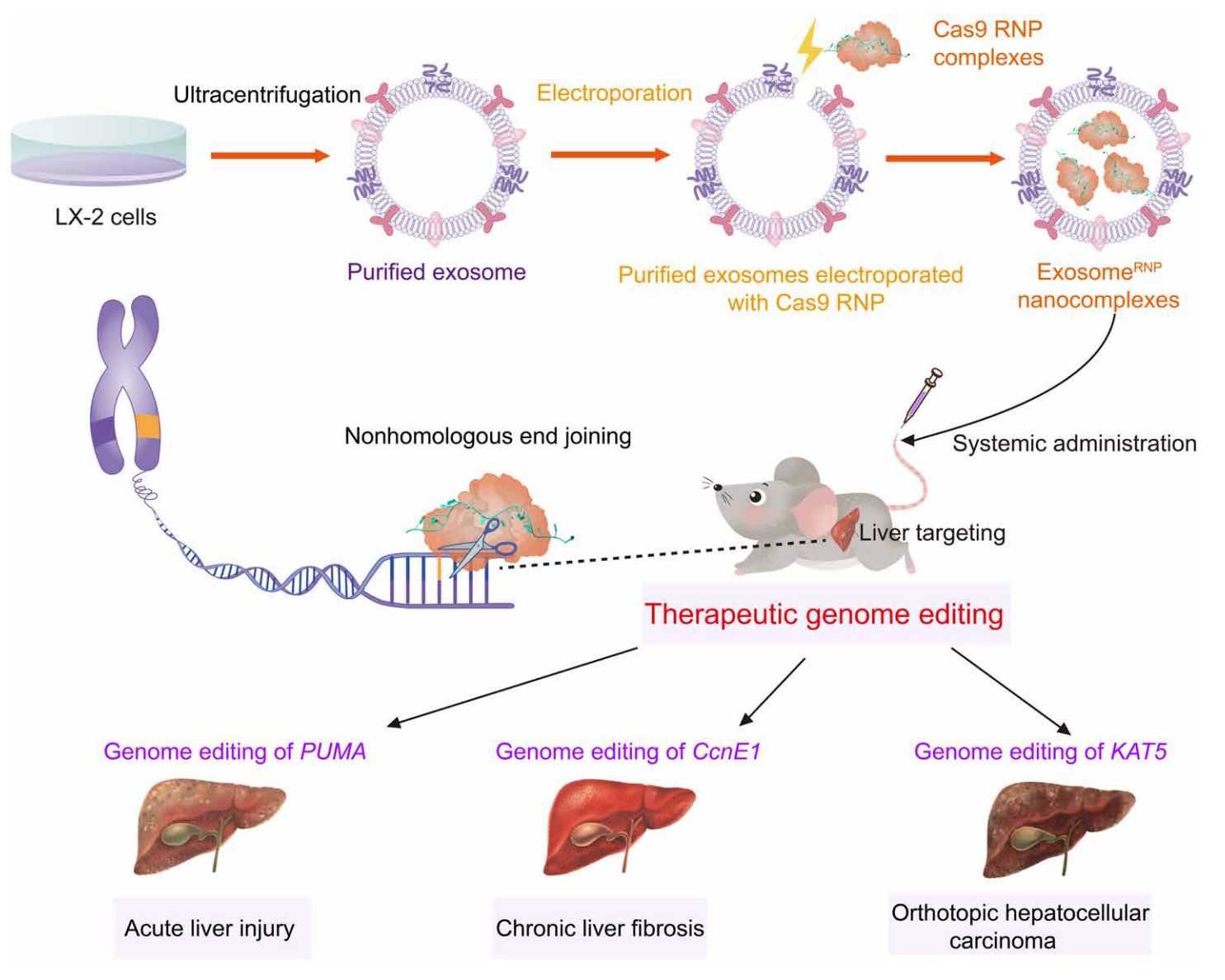 Figure 4. Schematic of exosome for delivery of Cas9 RNP to treat liver disorders. (Wan T, et al., 2022)
Figure 4. Schematic of exosome for delivery of Cas9 RNP to treat liver disorders. (Wan T, et al., 2022)
Posterior capsule opacification (PCO) is the most common complication after cataract surgery. Although drug-carrying artificial lenses (IOLs) have been successfully developed, their clinical effectiveness is still limited due to a lack of targeting and low bioavailability. In recent years, researchers have successfully developed an exosome-functionalized drug-carrying IOL that can effectively prevent PCO. Collection of exosomes from lens epithelial cells (LEC) are loaded with the antiproliferative drug adriamycin (Dox) by electroporation and then immobilized on the surface of aminated IOL by electrostatic action. In vitro experiments showed that the cellular uptake of Dox@Exos was significantly increased by LEC compared to free Dox, resulting in a better antiproliferative effect. Animal experiments showed that Dox@Exos-IOLs could effectively inhibit the development of PCO and had good intraocular biocompatibility.
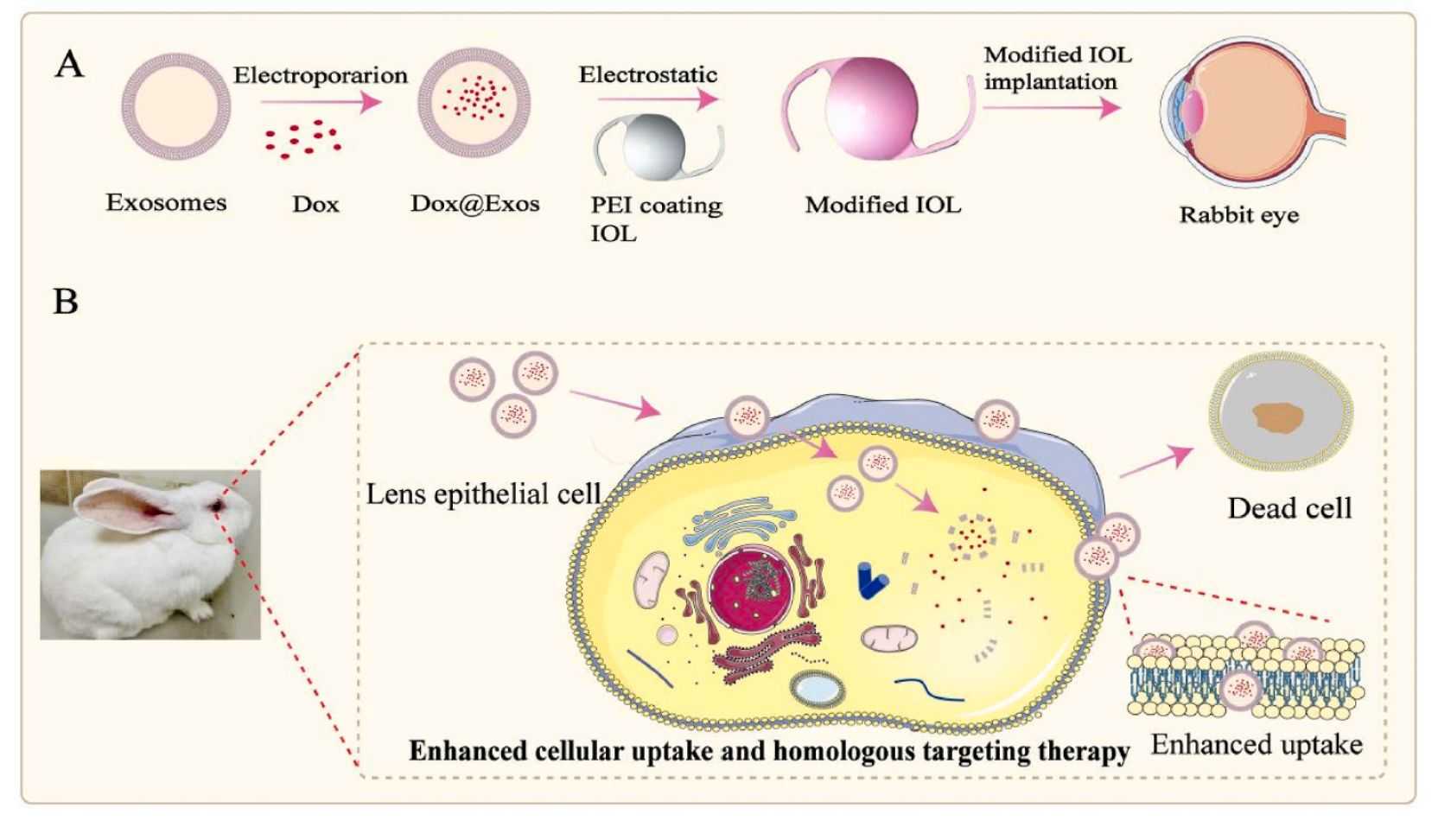 Figure 5. Fabrication of Dox@Exos modified IOLs and cellular uptake enhanced by homologous targeting for effective PCO prevention. (Zhu S, et al., 2022)
Figure 5. Fabrication of Dox@Exos modified IOLs and cellular uptake enhanced by homologous targeting for effective PCO prevention. (Zhu S, et al., 2022)
Our Products
We are a pioneering company dedicated to advancing exosome research and applications. Our services include electroporation-based exosome drug delivery designed to maximize the therapeutic potential of exosomes.
In addition to our cutting-edge delivery solutions, we offer a comprehensive range of high-quality exosome products that facilitate the research and exploration of exosomes as a powerful tool for drug delivery.
| Cat No. | Product Name | Source |
| Exo-CH11 | HQExo™ Exosome-K-562 | Exosome derived from human pleural effusion, leukemia chronic myelogenous (K-562 cell line) |
| Exo-CH17 | HQExo™ Exosome-HT29 | Exosome derived from human adenocarcinoma (HT29 cell line) |
| Exo-CH24 | HQExo™ Exosome-SK-BR-3 | Exosome derived from human breast carcinoma cell line (SK-BR-3) |
| Exo-SC03 | HQExo™ Exosome-hTERT | Exosome derived from hTERT-immortalized Mesenchymal Stem Cell |
| Exo-SC02-2 | HQExo™ Exosome-Pla-MSC | Exosome derived from human placental derived mesenchymal stem cell |
| Exo-IC02 | HQExo™ Exosome-JM1 | Exosome derived from human T pre-B lymphoblast cell line (JM1) |
| Exo-HDBF-02 | HQExo™ Exosome-SDH-Asthma plasma | Exosome derived from Single Donor Human Asthma plasma |
| Exo-HDBF-14 | HQExo™ Exosome-SDH-Bladder Cancer Plasma exosome | Exosome derived from Single Donor Human Bladder Cancer Plasma |
| PNE-FB04 | PNExo™ Exosome-Baby Kiwi | Exosome derived from Baby Kiwi |
| PNE-VA09 | PNExo™ Exosome-Artichokes | Exosome derived from Artichokes |
| Explore All Exosomes Products | ||
Creative Biostructure is a leader in the field of exosome research and our commitment is to support and accelerate clients' scientific endeavors by providing tailored, top-notch exosome solutions and high-quality exosome products. Please feel free to contact us for a formal quote.
References
- Xu Y, et al. Tumor-derived extracellular vesicles as messengers of natural products in cancer treatment. Theranostics. 2022. 12(4): 1683-1714.
- Du X, et al. Advanced physical techniques for gene delivery based on membrane perforation. Drug Deliv. 2018. 25(1): 1516-1525.
- Lennaárd AJ, et al. Optimized Electroporation for Loading of Extracellular Vesicles with Doxorubicin. Pharmaceutics. 2021. 14(1): 38.
- Wan T, et al. Exosome-mediated delivery of Cas9 ribonucleoprotein complexes for tissue-specific gene therapy of liver diseases. Sci Adv. 2022. 8(37): eabp9435.
- Zhu S, et al. Augmented cellular uptake and homologous targeting of exosome-based drug loaded IOL for posterior capsular opacification prevention and biosafety improvement. Bioact Mater. 2022. 15: 469-481.
- Zeng H, et al. Current Strategies for Exosome Cargo Loading and Targeting Delivery. Cells. 2023. 12(10): 1416.