Structural Research of CorA Superfamily Ion Transporters
The CorA superfamily ion transporters play an essential role in maintaining ion homeostasis in bacteria. These proteins have a membrane domain in series with a cytoplasmic domain that together form a continuous channel. Recent advances in structural research have provided valuable insight into the molecular mechanisms underlying ion transport in the CorA superfamily, with implications for the development of new antimicrobial agents that target bacterial ion transport systems.
X-ray crystallography has been used to determine the three-dimensional structure of the cytosolic domain of the CorA Mg2+ channel, which is responsible for binding and regulating the transport of magnesium ions across the bacterial cell membrane. Structural analysis has identified an Mg2+-ligand binding site located in a novel position between each of the five subunits and two Mg2+ ions trapped inside the pore. Further research has also demonstrated the importance of cooperativity in the function of the CorA Mg2+ transporter, with multiple copies of the cytosolic domain within the channel interacting with each other to facilitate magnesium ion transport.
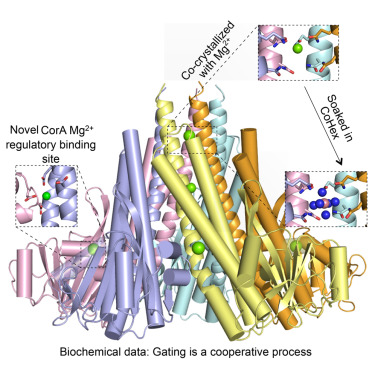 Figure 1. Structure and cooperativity of the cytosolic domain of the CorA Mg2+ channel from E. coli. (Lerche M, et al., 2017)
Figure 1. Structure and cooperativity of the cytosolic domain of the CorA Mg2+ channel from E. coli. (Lerche M, et al., 2017)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| CorA Mg2+ Transporter (expressed in E. coli) | Thermotoga maritima | X-ray diffraction | 3.90 Å | 2BBJ |
| CorA Mg2+ Transporter (expressed in E. coli) | Thermotoga maritima | X-ray diffraction | 2.90 Å | 2IUB |
| CorA Mg2+ transporter homologue (expressed in E. coli) | Thermotoga maritima | X-ray diffraction | 3.70 Å | 2HN2 |
| CorA coiled-coil mutant under Mg2+ absence (expressed in E. coli) | Thermotoga maritima | X-ray diffraction | 3.80 Å | 4EEB |
| CorA Mg2+ transporter in the closed symmetric magnesium-bound state (expressed in E. coli) | Thermotoga maritima | Cryo-EM single particle analysis | 3.80 Å | 3JCF |
| CorA Mg2+ Transporter (expressed in E. coli) | Methanocaldococcus jannaschii | X-ray diffraction | 3.20 Å | 4EV6 |
| CorA Mg2+ Transporter cytoplasmic domain with bound Mg2+ (expressed in E. coli) | Escherichia coli | X-ray diffraction | 2.80 Å | 5N77 |
| ZntB Zn2+ transporter cytoplasmic domain (expressed in E. coli) | Vibrio parahaemolyticus | X-ray diffraction | 1.90 Å | 3CK6 |
| Soluble domain structure of the ZntB Zn2+ efflux system (expressed in E. coli) | Salmonella enterica | X-ray diffraction | 2.30 Å | 3NVO |
Table 1. Structural Research of CorA Superfamily Ion Transporters.
Creative Biostructure is a preeminent provider of innovative protein structure analysis services, which exploit the full potential of advanced structural biology techniques to investigate the structure and function of membrane proteins. Our team of scientists possesses extensive experience in X-ray crystallography, cryo-electron microscopy, and NMR spectroscopy. This endows us with the capability to provide an exhaustive repertoire of structural analysis services for membrane proteins.
Our membrane protein structure analysis service proffers invaluable assistance to researchers in unraveling the complex molecular mechanisms underlying ion transport in CorA superfamily ion transporters. Owing to our unparalleled expertise in structural biology, we provide accurate and detailed insights into the structure and function of these indispensable membrane proteins. Such insights furnish researchers with an enhanced comprehension of the molecular basis of bacterial ion homeostasis, thereby facilitating the development of novel antimicrobial agents that are tailored to target bacterial ion transport systems.
If you harbor any inclination towards gaining further knowledge regarding our services or would like to engage in a discussion with one of our proficient scientists regarding your project, please do not hesitate to contact us. Our team of preeminent scientists is always on standby, eager to discuss your research requirements and provide you with the optimal solutions for your project.
References
- Lerche M, et al. Structure and cooperativity of the cytosolic domain of the CorA Mg2+ channel from Escherichia coli. Structure. 2017, 25(8): 1175-1186. e4.
- Wan Q, et al. X-ray crystallography and isothermal titration calorimetry studies of the Salmonella zinc transporter ZntB. Structure. 2011, 19(5): 700-710.