Exosomes for lncRNA Delivery
Long non-coding RNA (lncRNA) is a type of RNA that is more than 200nt in length and does not have any protein-coding ability. Recent research has found that lncRNAs play essential roles in the control of gene expression, regulating critical genes involved in cancer development. Therefore, lncRNAs can be used as new tools for cancer therapy. However, lncRNA-based drugs are clinically limited. Unlike other non-coding RNAs (e.g. microRNAs), most lncRNAs have high molecular weights and conserved secondary structures, which complicate the delivery of lncRNAs. Since lncRNAs are the most abundant part of the mammalian genome, further exploration of lncRNA delivery and subsequent functional research is essential for potential clinical applications.
What is LncRNA?
LncRNAs play essential regulatory roles in developmental and pathological processes and contribute to a variety of biological processes, including cell cycle regulation, immune surveillance, and embryonic stem cell pluripotency. Recent research has also shown that lncRNAs are involved in many important regulatory processes, such as X-chromosome silencing, genomic imprinting and chromatin modification, transcriptional activation and disruption, and nuclear translocation. LncRNAs can be localized in the nucleus or cytoplasm of cells. It has been demonstrated that lncRNAs can serve as scaffolds for assembling multicomponent complexes. Depending on the subcellular localization of lncRNAs, they directly regulate gene expression by:
a) Binding to chromatin regulatory proteins to influence chromatin modification.
b) Regulating mRNA splicing and stability.
c) Indirectly participating in transcriptional and post-transcriptional gene expression mechanisms through interactions with other RNAs and proteins.
In addition, several reports have emphasized the interaction between lncRNAs and miRNAs. LncRNAs can cause re-expression of miRNA target genes by sequestering miRNAs and acting as decoys.
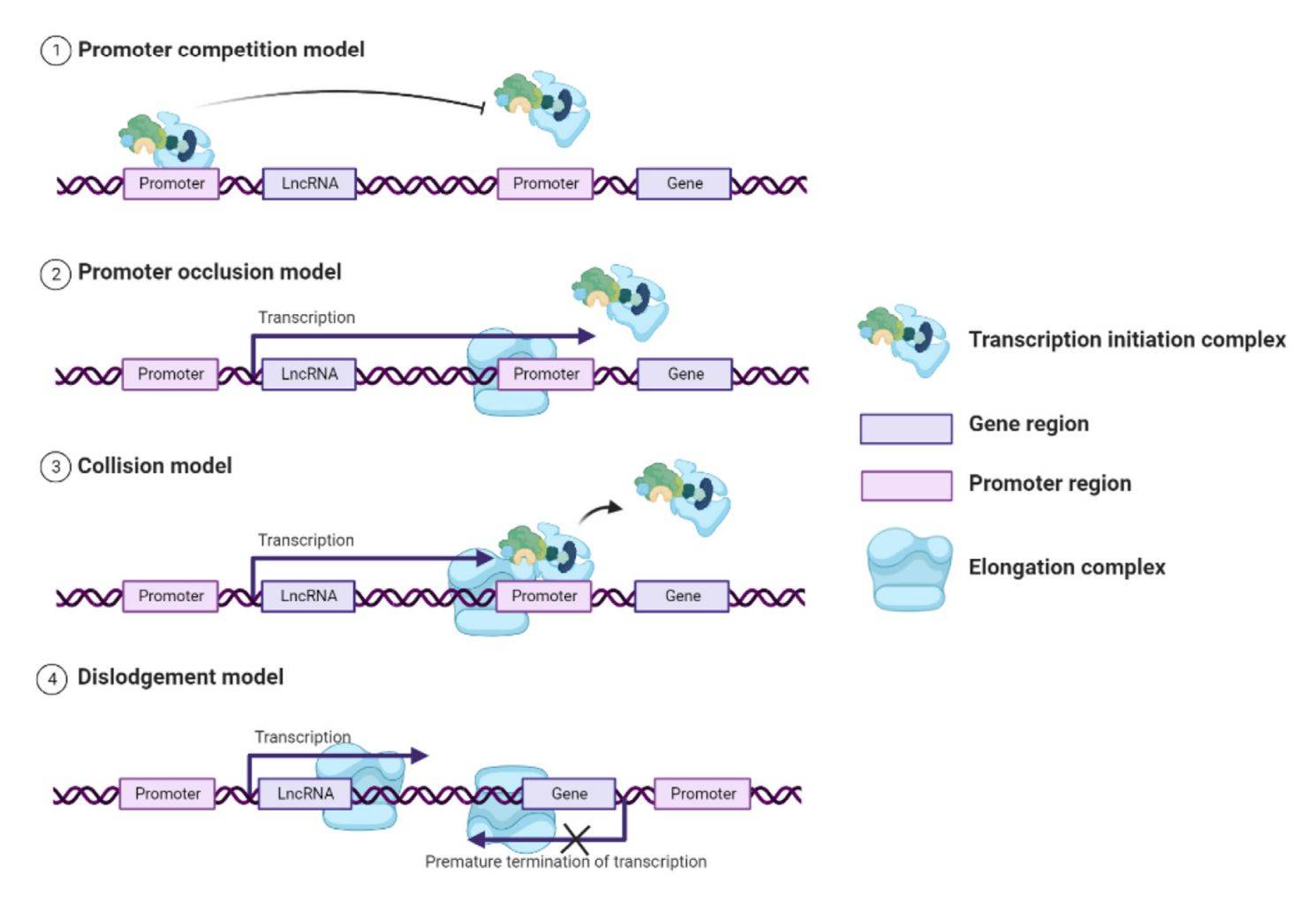 Figure 1. LncRNAs perform transcriptional interference by directly repressing the transcription of a second gene through multiple modes of action. (Han S, et al., 2023)
Figure 1. LncRNAs perform transcriptional interference by directly repressing the transcription of a second gene through multiple modes of action. (Han S, et al., 2023)
What are the Benefits of Exosomes for lncRNA Delivery?
Exosomes are phospholipid nanovesicles of extracellular origin that transport bioactive molecules to specific receptor cells and are regarded as natural nanocarriers for lncRNA-based therapeutic delivery. Exosomes have unique advantages over conventional delivery systems.
- Exosomes have low cytotoxicity and immunogenicity since they are natural carriers of cargoes including proteins, nucleic acids, and lipids, and have better biocompatibility due to membrane proteins such as tetraspanins and fibronectin.
- Exosomes circulate in the body for longer periods and exhibit greater stability in plasma. They are not easily cleared by macrophages or reticuloendothelial cells.
- Exosomes can cross the blood-brain barrier (BBB) and efficiently deliver cargo to the central nervous system.
- Through genetic engineering methods such as transfection, it is possible to express homing peptides or ligands on exosome membranes to improve targeting and therapeutic efficiency.
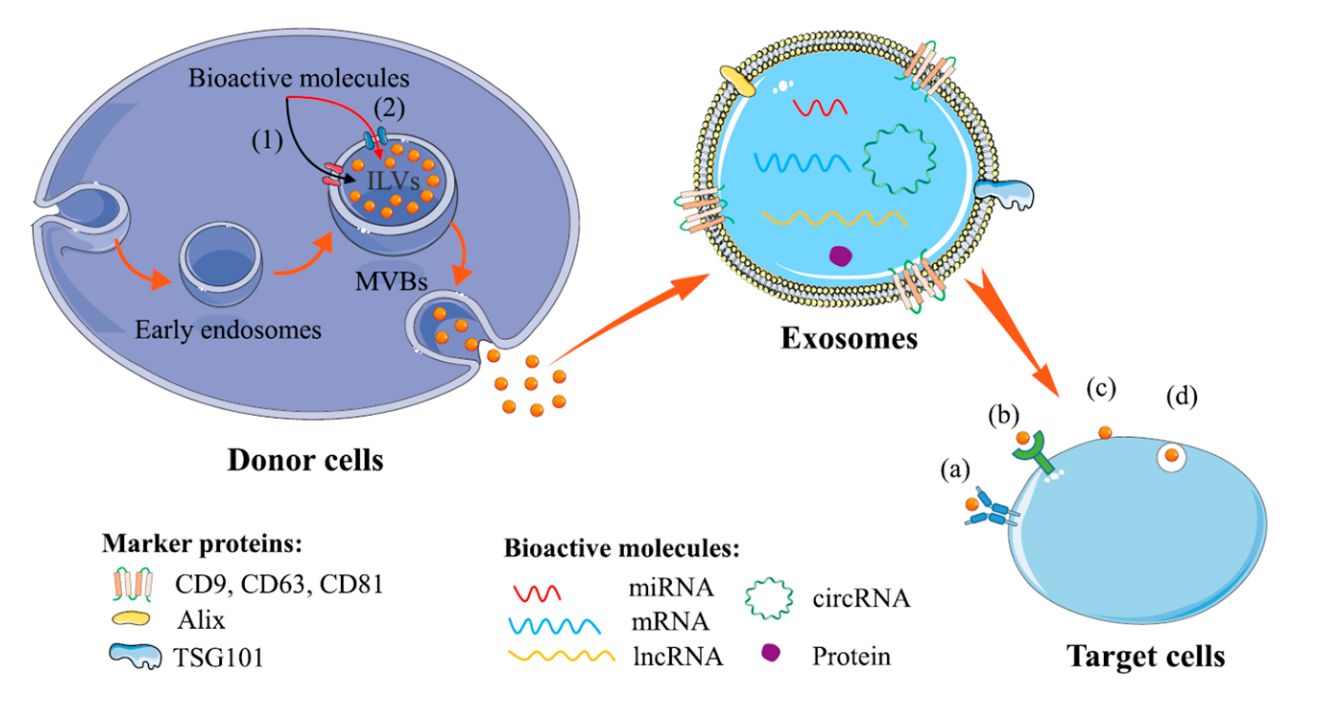 Figure 2. Overview of exosome biogenesis. (Kar R, et al., 2023)
Figure 2. Overview of exosome biogenesis. (Kar R, et al., 2023)
Application Cases of Exosome Delivery of lncRNAs
Diabetic wounds are one of the most debilitating complications of diabetes, and hyperglycemia-induced vascular insufficiency is a major cause of impaired diabetic wound healing. Recently, LncRNA-H19 has attracted increasing attention for its potential to trigger angiogenesis, which is significantly reduced in diabetes. Therefore, therapeutic strategies utilizing LncRNA-H19 delivery are feasible. Researchers used extracellular vesicle-mimicking nanovesicles (EMNV) as a nanodrug delivery vehicle for lncRNA. The results showed that bioengineered EMNV can be used as a powerful tool for delivering LncRNA-H19 with a strong ability to neutralize the regenerative inhibitory effects of hyperglycemia and can significantly accelerate the healing process of chronic wounds.
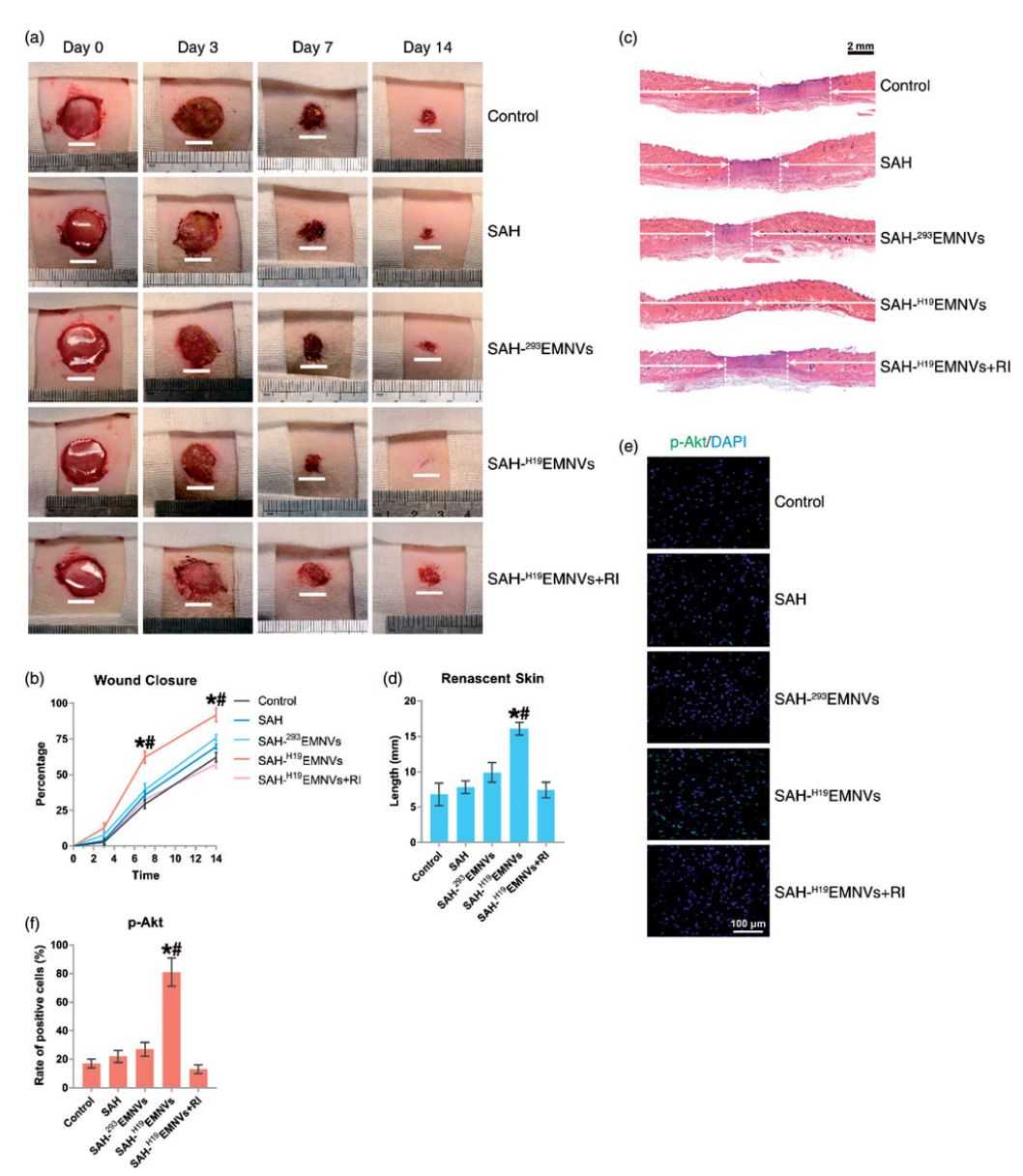 Figure 3. H19EMNVs promoted the healing of diabetic wounds. (Tao SC, et al., 2018)
Figure 3. H19EMNVs promoted the healing of diabetic wounds. (Tao SC, et al., 2018)
Exosomes are nano-sized membrane vesicles that can be targeted to deliver therapeutic agents by modifying targeting ligands. Previous evidence suggests that long-chain non-coding RNA maternally expressed gene 3 (lncRNA MEG3) has anti-tumor properties. Researchers used engineering techniques to combine exosomes and lncRNAs for tumor-targeted therapy of osteosarcoma. In addition, they prepared c(RGDyK)-modified and MEG3-loaded exosomes (cRGD-Exo-MEG3), which can deliver drugs to OS cells more efficiently in vivo and in vitro. Research has shown that cRGD-Exo-MEG3 significantly enhances the anti-osteosarcoma effect of MEG3 and enhances tumor-targeted therapy. Engineered exosomes are potentially therapeutic for osteosarcoma as delivery vehicles for targeting lncRNA MEG3.
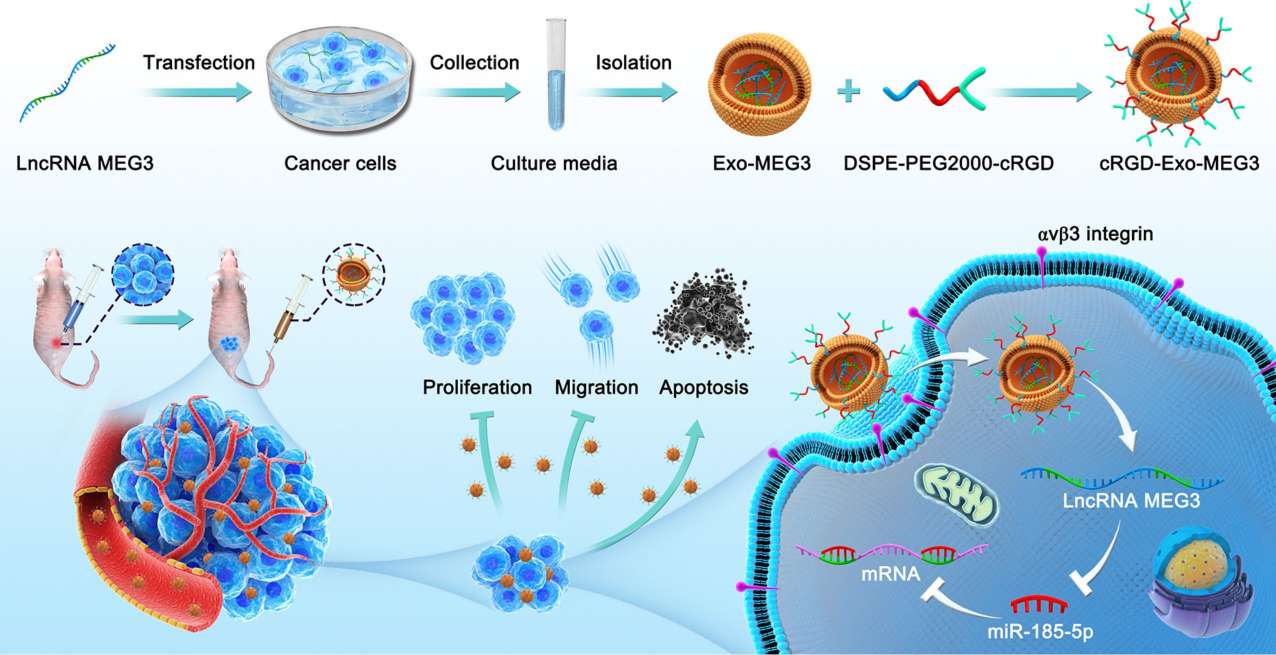 Figure 4. Exosome as targeted lncRNA MEG3 delivery vehicles for osteosarcoma therapy. (Huang X, et al., 2022)
Figure 4. Exosome as targeted lncRNA MEG3 delivery vehicles for osteosarcoma therapy. (Huang X, et al., 2022)
Our Products
Several lncRNA-based drugs have entered clinical trials and shown promising results in tumor therapy. To ensure biological function, the transfer of lncRNA must maintain its secondary structure to ensure activity. As a naturally mediated carrier, exosomes are the most ideal carriers for lncRNA-based drugs.
In addition to providing highly efficient exosome engineering services (including exosome cargo loading), we offer a range of high-quality exosomes isolated from cancer cell lines to help clients explore exosome-based anticancer drug delivery strategies.
| Cat No. | Product Name | Source |
| Exo-CH03 | HQExo™ Exosome-DMS114 | Exosome derived from human small cell lung cancer cell line (DMS114 cell line) |
| Exo-CH08 | HQExo™ Exosome-BPH-1 | Exosome derived from human benign prostatic hyperplasia-1 (BPH-1 cell line) |
| Exo-CH10 | HQExo™ Exosome-HCT116 | Exosome derived from human colorectal carcinoma cell line (HCT116 cell line) |
| Exo-CH12 | HQExo™ Exosome-MM1 | Exosome derived from human melanoma (MM1 cell line) |
| Exo-CH17 | HQExo™ Exosome-HT29 | Exosome derived from human adenocarcinoma (HT29 cell line) |
| Exo-CH20 | HQExo™ Exosome-COLO205 | Exosome derived from human colon carcinoma (COLO205 cell line) |
| Exo-CH24 | HQExo™ Exosome-SK-BR-3 | Exosome derived from human breast carcinoma cell line (SK-BR-3) |
| Explore All Exosomes Isolated from Cancer Cell Lines | ||
With experienced experts and cutting-edge equipment, Creative Biostructure provides one-stop exosome services, including exosome isolation, characterization, labeling, tracking, targeting, and functional analysis, to help clients explore the potential applications of exosomes. Please feel free to contact us for a formal quote.
References
- Han S, et al. The tumor therapeutic potential of long non-coding RNA delivery and targeting. Acta Pharm Sin B. 2023. 13(4): 1371-1382.
- Kar R, et al. Exosome-Based Smart Drug Delivery Tool for Cancer Theranostics. ACS Biomater Sci Eng. 2023. 9(2): 577-594.
- Tao SC, et al. Extracellular vesicle-mimetic nanovesicles transport LncRNA-H19 as competing endogenous RNA for the treatment of diabetic wounds. Drug Deliv. 2018. 25(1): 241-255.
- Huang X, et al. Engineered exosome as targeted lncRNA MEG3 delivery vehicles for osteosarcoma therapy. J Control Release. 2022. 343: 107-117.
- Ratti M, et al. MicroRNAs (miRNAs) and Long Non-Coding RNAs (lncRNAs) as New Tools for Cancer Therapy: First Steps from Bench to Bedside. Target Oncol. 2020. 15(3): 261-278.