Structural Research of Toll-Like Receptors (TLR) and TLR Signalling Regulators
Membrane-bound Toll-like receptors (TLRs) trigger innate immune responses upon recognition of a variety of pathogen-derived compounds, and recognition of invading pathogens is essential for initiating antimicrobial responses and triggering adaptive immunity. Research has found that activation of the TLR induces pro-inflammatory cytokines that trigger the synthesis of inflammatory mediators, leading to fever, pain, and other inflammatory conditions, followed by type I interferon-mediated antiviral responses. In recent years, researchers have analyzed the function of TLR from the structural level, which is of great significance for analyzing its action mechanisms involved in cellular responses.
Advances in research on TLR structure
TLR belongs to the type I integral transmembrane proteins, which usually contains an N-terminal domain (NTD) located outside the membrane, an intermediate single-helix transmembrane domain that crosses the membrane, and a C-terminal domain (CTD) located in the cytoplasm. The NTD constitutes an extracellular domain that serves as a ligand recognition site for various PAMPs, and the CTD is involved in interactions with various signaling junctions, initiating downstream signaling homology domains through its toll-IL-1 receptor (TIR). In addition, the NTD contains glycan portions that serve as the actual binding sites for various pathogen-derived ligands.
General structural analysis of TLR extracellular domains
The first reported crystal structure of the TLR extracellular domain (TLR-ECD) is the unliganded form of TLR3. This structure provides the general structural architecture of the TLR extracellular domain. TLR-ECDs share a common structural framework and adopt a horseshoe structure built from leucine-rich repeat (LRR) motifs. The N-terminal and C-terminal ends of TLR-ECDs are capped with LRR-NT and LRR-CT motifs. LRR-NT is a disulfide-bonded β-hairpin, whereas LRR-CT is a globular structure containing two α-helices stabilized by two disulfide bonds.
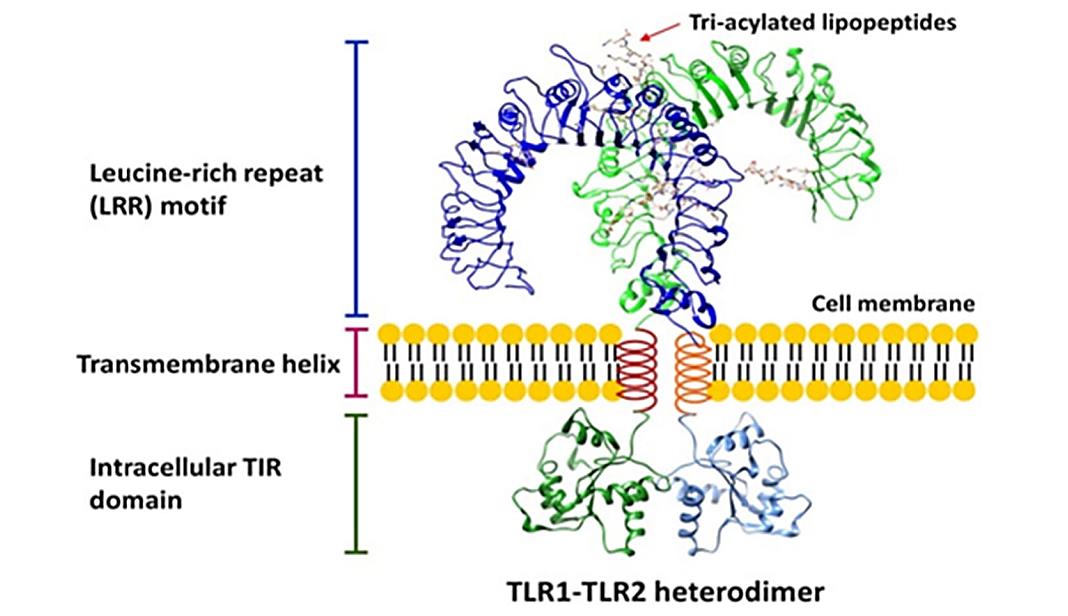 Figure 1. The overall structure of the TLR. (El-Zayat, S. R., et al., 2019)
Figure 1. The overall structure of the TLR. (El-Zayat, S. R., et al., 2019)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| The TIR domain of Toll-Like Receptor 10 (TLR10) | Homo sapiens | X-ray diffraction | 2.2 Å | 2J67 |
| TIR domain of TLR6 | Homo sapiens | X-ray diffraction | 2.204 Å | 4OM7 |
| Toll-like receptor 1 (TLR1) TIR domain | Homo sapiens | SOLUTION NMR | / | 7NT7 |
| TIR domain of TLR1 (crystallized with Zn2+ ions) | Homo sapiens | X-ray diffraction | 1.9 Å | 7NUW |
| TIR domain of TLR1 (crystallized without ZN2+ ions) | Homo sapiens | X-ray diffraction | 2.47 Å | 7NUX |
| Cryptic TIR | Homo sapiens | X-ray diffraction | 2.5 Å | 5FOR |
| TcpB and the TLR adaptor protein TIRAP | Brucella melitensis | X-ray diffraction | 2.3 Å | 4LQC |
| Toll-like receptor 15 TIR domain (glutathione adduct) | Gallus gallus | X-ray diffraction | 1.8 Å | 7YLG |
| Toll-like receptor 15 TIR domain (2-mercaptoethanol adduct) | Gallus gallus | X-ray diffraction | 1.9 Å | 7YLF |
| TLR7 in complex with UNC93B1 | Homo sapiens | Cryo-EM single particle analysis | 4.2 Å | 7CYN |
| TLR3 in complex with UNC93B1 | Homo sapiens | Cryo-EM single particle analysis | 3.4 Å | 7C76 |
| SLC15A4_TASL complex | Homo sapiens | Cryo-EM single particle analysis | 3.05 Å | 8JZU |
| The dimeric transmembrane domain of Toll-like receptor 3 | Homo sapiens | SOLUTION NMR | / | 2MK9 |
| Toll-like receptor 3 transmembrane domain in the trimeric state | Homo sapiens | SOLUTION NMR | / | 2MKA |
| N-terminal domain of Toll receptor | Drosophila melanogaster | X-ray diffraction | 2.41 Å | 4ARN |
| TLR8 in complex with DS-877 | Homo sapiens | X-ray diffraction | 1.81 Å | 3WN4 |
| TLR8 in complex with CU-CPD107 | Homo sapiens | X-ray diffraction | 2.89 Å | 7CRF |
| TLR8 in complex with XG-1-236 | Homo sapiens | X-ray diffraction | 2.1 Å | 4QC0 |
| TLR8 in complex with DS-802 | Homo sapiens | X-ray diffraction | 2 Å | 4QBZ |
| TLR3 in complex with UNC93B1 | Mus musculus | Cryo-EM single particle analysis | 3.3 Å | 7C77 |
Table 1. Structural research of the Toll-like receptors (TLR) and TLR signaling regulators.
Creative Biostructure is an industry leader in providing structural analysis services. With a team of outstanding experts specializing in the use of advanced technologies such as X-ray crystallography, cryo-electron microscopy (cryo-EM), and NMR spectroscopy, we can provide an in-depth understanding of the complex structure and function of Toll-like receptors (TLRs) and TLR signaling regulators.
With our unwavering commitment to delivering high-quality, accurate, and timely structural analysis results, clients benefit from our comprehensive structural analysis services, from protein expression and purification to structure determination. Our strong dedication to quality and excellence expertise and experience makes us the first choice to satisfy all your structural analysis requirements. If you have any questions, please feel free to contact us.
References
- El-Zayat, S. R., et al. Toll-like receptors activation, signaling, and targeting: an overview. Bulletin of the National Research Centre.2019. 43(1): 187.
- Sameer AS, Nissar S. Toll-Like Receptors (TLRs): Structure, Functions, Signaling, and Role of Their Polymorphisms in Colorectal Cancer Susceptibility. Biomed Res Int. 2021. 2021: 1157023.
- Asami J, Shimizu T. Structural and functional understanding of the toll-like receptors. Protein Sci. 2021. 30(4): 761-772.
