Labeling Exosomes with Lipophilic Dyes
Exosome labeling plays a critical role in researching and analyzing these extracellular vesicles. Researchers can track the movement of exosomes, observe interactions with other cells, monitor their uptake and release, and understand the function of exosomes in biological processes. In addition, exosome labeling makes it possible to quantify exosomes, which contributes to their utilization in biomedical applications, such as therapeutic drug delivery.
Fluorescent labeling allows imaging and tracking of vesicles down to the single particle level. Among several options for introducing fluorescence, staining lipid membranes with lipophilic dyes provides a simple method without disturbing vesicle content. Exosomes, vesicles enclosed in a lipid bilayer, provide an ideal binding environment for these dyes. Lipophilic dyes cross the lipid bilayer of exosomes and covalently bind to their components, causing them to fluoresce. This allows exosomes to be detected and observed under a fluorescence microscope, thus facilitating their research and understanding.
Why can Exosomes be Labeled with Lipophilic Dyes?
Exosomes are cellular vesicles with a diameter of about 30-150 nm secreted by living cells and have a typical lipid bilayer structure. Lipophilic dyes, such as lipophilic carbocyanine dyes or lipophilic PKH dyes, have a high affinity for the lipid bilayer and can easily bind to the exosome membrane, resulting in stable and reliable labeling of exosomes for various downstream analyses. This property of lipophilic dyes makes them suitable for tracking and researching exosomes in a variety of biological systems and experimental settings. In addition, the labeling process of these dyes is relatively simple and does not require complex procedures. This makes lipophilic dyes a practical choice for researchers studying exosomes.
What are the Lipophilic Dyes Commonly Used to Label Exosomes?
Most of the published articles on exosomes have been labeled with lipophilic dyes, which have been used more often both in vivo and in vitro. Lipophilic dyes are divided into two main categories:
- PKH Lipophilic Dyes
PKH dyes are highly fluorescent and their aliphatic tails rapidly insert into the exposed lipid bilayer and form strong non-covalent interactions, thus promoting long-term dye retention and stable fluorescence. PKH dyes have been successfully used to label exosomes and extracellular vesicles in tracer assays.PKH dyes are generally characterized by low toxicity, low fluorescence background, high lipid solubility, high penetration, and strong and stable fluorescence. The dye can be easily detected by flow cytometry and fluorescence microscopy after a simple and rapid staining procedure.
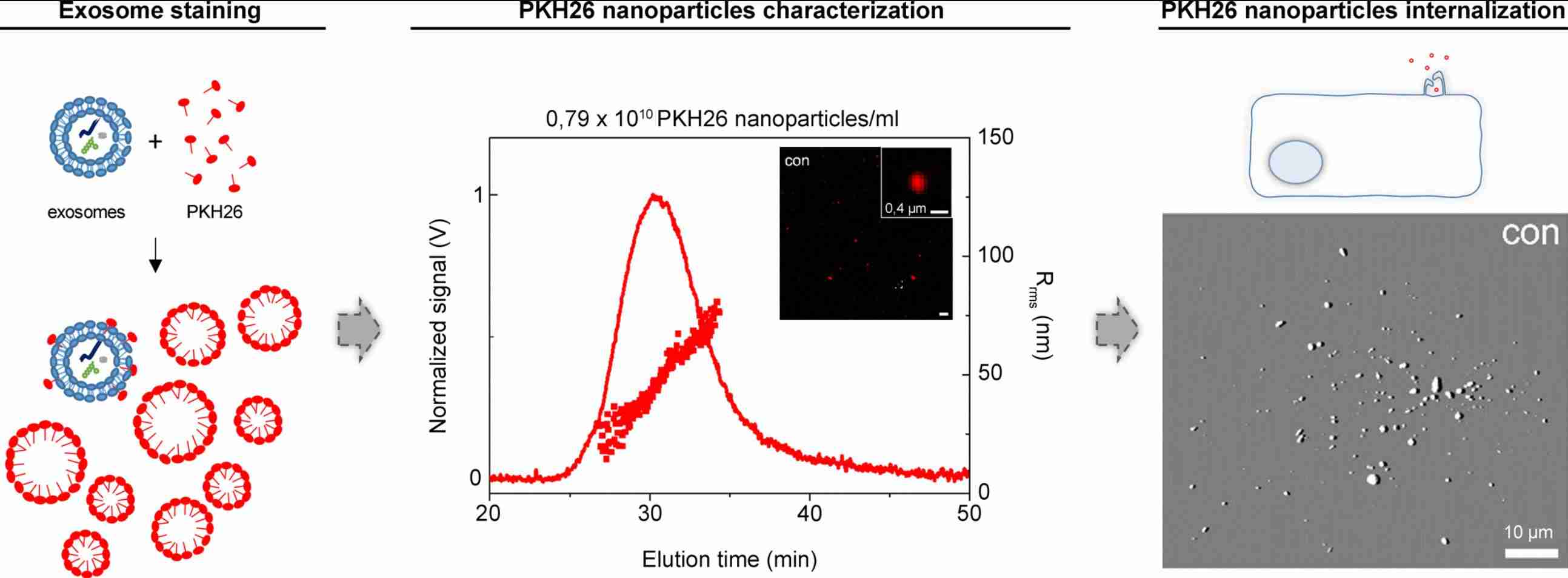 Figure 1. Labeling exosomes with PKH26 fluorescent dye. (Pužar Dominkuš P, et al., 2018)
Figure 1. Labeling exosomes with PKH26 fluorescent dye. (Pužar Dominkuš P, et al., 2018)
- Lipophilic Carbocyanine Dyes
Carbocyanine dyes are widely used to label exosomes, cells, organelles, liposomes, viruses, and lipoproteins. Long-chain carbocyanines include DiO (DiOC18 (3)), DiI (DiIC18 (3)), DiD (DiIC18 (5)), and DiR, as well as the dialkylaminostyryl dye, DiA (4-Di-16-ASP), which is used to label membranes and other hydrophobic structures. They have high extinction coefficients, environment-dependent fluorescence, and short excited state lifetimes in lipid environments. Carbocyanine dyes are oily at room temperature and emit weak fluorescence in water, but when doped into membranes or bound to lipophilic biomolecules, they emit high fluorescence and are quite lightfast. These optical properties make them ideal for exosome staining.
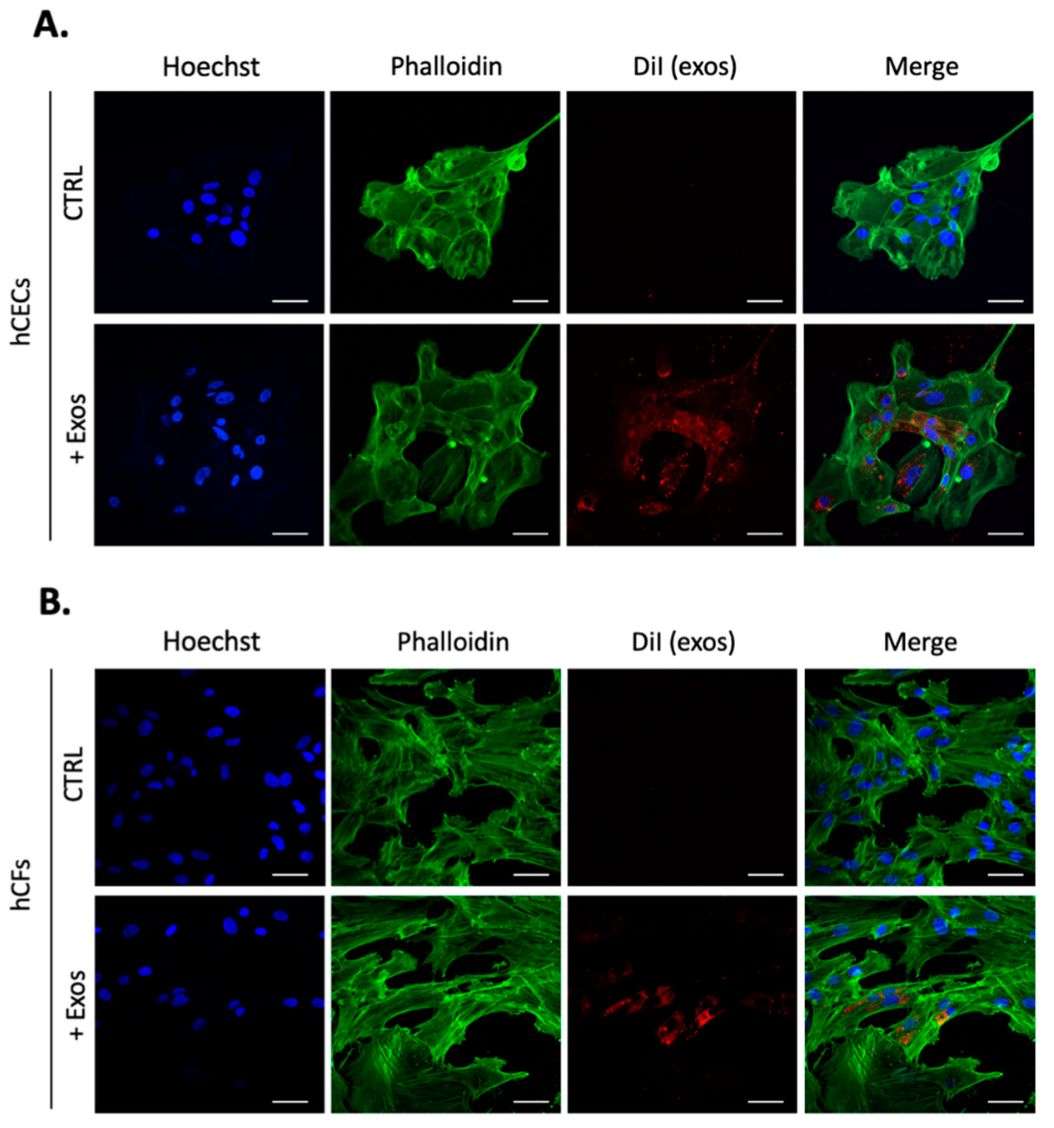 Figure 2. Exosomes uptake in hCECs and hCFs labeled with lipophilic dye DiI. (Desjardins P, et al., 2022)
Figure 2. Exosomes uptake in hCECs and hCFs labeled with lipophilic dye DiI. (Desjardins P, et al., 2022)
What Research Advances Have Been Made in Labeling Exosomes with Lipophilic Dyes?
Exosome staining with lipophilic dyes is often affected by confounding factors, such as lipoprotein off-target labeling and complications resulting from free dye aggregates similar in size to exosomes. In addition, research has shown that the incorporation of lipophilic dyes may also lead to a significant increase in exosome size. Therefore, researchers propose an improved method for fluorescently labeling exosomes with high sensitivity, specificity, and simplicity to maintain sample quality and quantity.
By characterizing the aggregation state of the lipophilic tracer DiI as a function of NaCl concentration, using total internal reflection fluorescence (TIRF) microscopy, it was observed that ~150 mM NaCl induces significant aggregation of DiI molecules, which could potentially inhibit their entry into the exosome membrane. This method is simple, rapid, and effective in improving labeling efficiency and removing unwanted free dye.
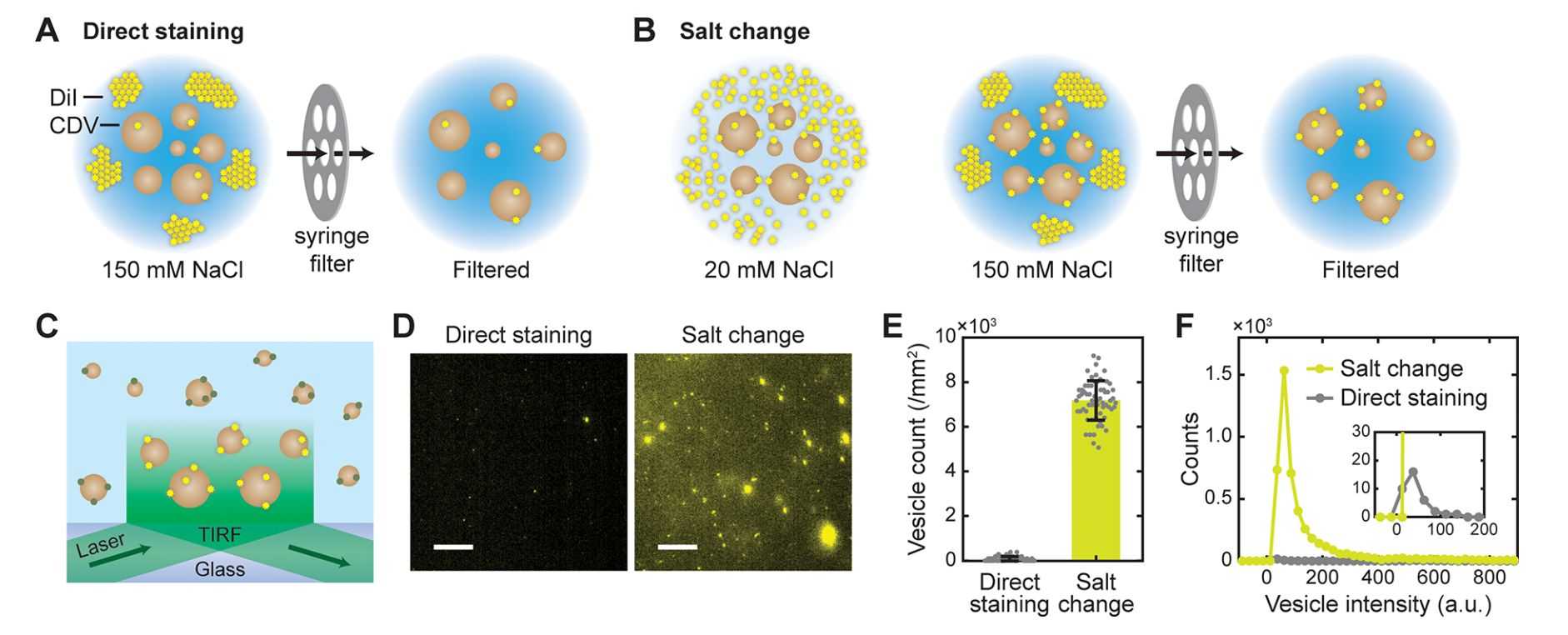 Figure 3. Fluorescent labeling of vesicles by salt-change method. (Cha M, et al., 2023)
Figure 3. Fluorescent labeling of vesicles by salt-change method. (Cha M, et al., 2023)
What are the Advantages of Labeling Exosomes with Lipophilic Dyes?
- Visualization - Lipophilic dyes help display exosomes under a fluorescence microscope or flow cytometer, allowing researchers to track their uptake, distribution, and behavior in recipient cells in real-time.
- Tracking - By labeling exosomes with lipophilic dyes, researchers can track the movement and fate of exosomes to research their biodistribution, pharmacokinetics, and targeting efficiency in a variety of biological systems.
- Quantification - Lipophilic dyes can be used to quantify the amount of exosomes released by cells and the level of exosome uptake by recipient cells, providing valuable insights into exosome biology and function.
- Cell-Specific Labeling - Certain lipophilic dyes can be conjugated to target ligands or antibodies for cell-specific labeling of exosomes, allowing researchers to study cell type-specific exosome uptake and signaling pathways.
- Stability and Versatility - Lipophilic dyes are known for their stability and versatility in labeling a wide variety of biological structures, making them suitable for long-term follow-up and multiple labeling experiments involving exosomes.
Lipophilic fluorescent dyes can be used for in vivo and in vitro functional studies of exosomes, such as exosome labeling and exosome tracking services. Creative Biostructure develops the most appropriate exosome research protocols according to the client's research objectives. Please do not hesitate to contact us for more information.
References
- Pužar Dominkuš P, et al. PKH26 labeling of extracellular vesicles: Characterization and cellular internalization of contaminating PKH26 nanoparticles. Biochim Biophys Acta Biomembr. 2018. 1860(6): 1350-1361.
- Desjardins P, et al. Impact of Exosomes Released by Different Corneal Cell Types on the Wound Healing Properties of Human Corneal Epithelial Cells. International Journal of Molecular Sciences. 2022. 23(20): 12201.
- Cha M, et al. Efficient Labeling of Vesicles with Lipophilic Fluorescent Dyes via the Salt-Change Method. Anal Chem. 2023. 95(14): 5843-5849.