Exosomes Loading Drugs by Preloading
Exosomes are star molecules in extracellular vesicles, which can carry and transfer a variety of biological information to cells at a distance, achieving the purpose of intercellular information transfer. Currently, scholars have introduced exosomes into the field of drug delivery research. As a potential drug delivery system, exosomes are similar to liposomes with a lipid bilayer membrane wrapped around a hydrophilic core that can be loaded with hydrophilic and lipophilic materials. In addition, exosomes have tissue and cellular targeting, better biocompatibility, low immunogenicity, and low toxicity, and can avoid recognition by the immune system. An essential part of drug delivery system (DDS) design is the selection of appropriate drug-loading methods. Currently, the loading of cargoes into exosomes has been successfully achieved using preloading strategies.
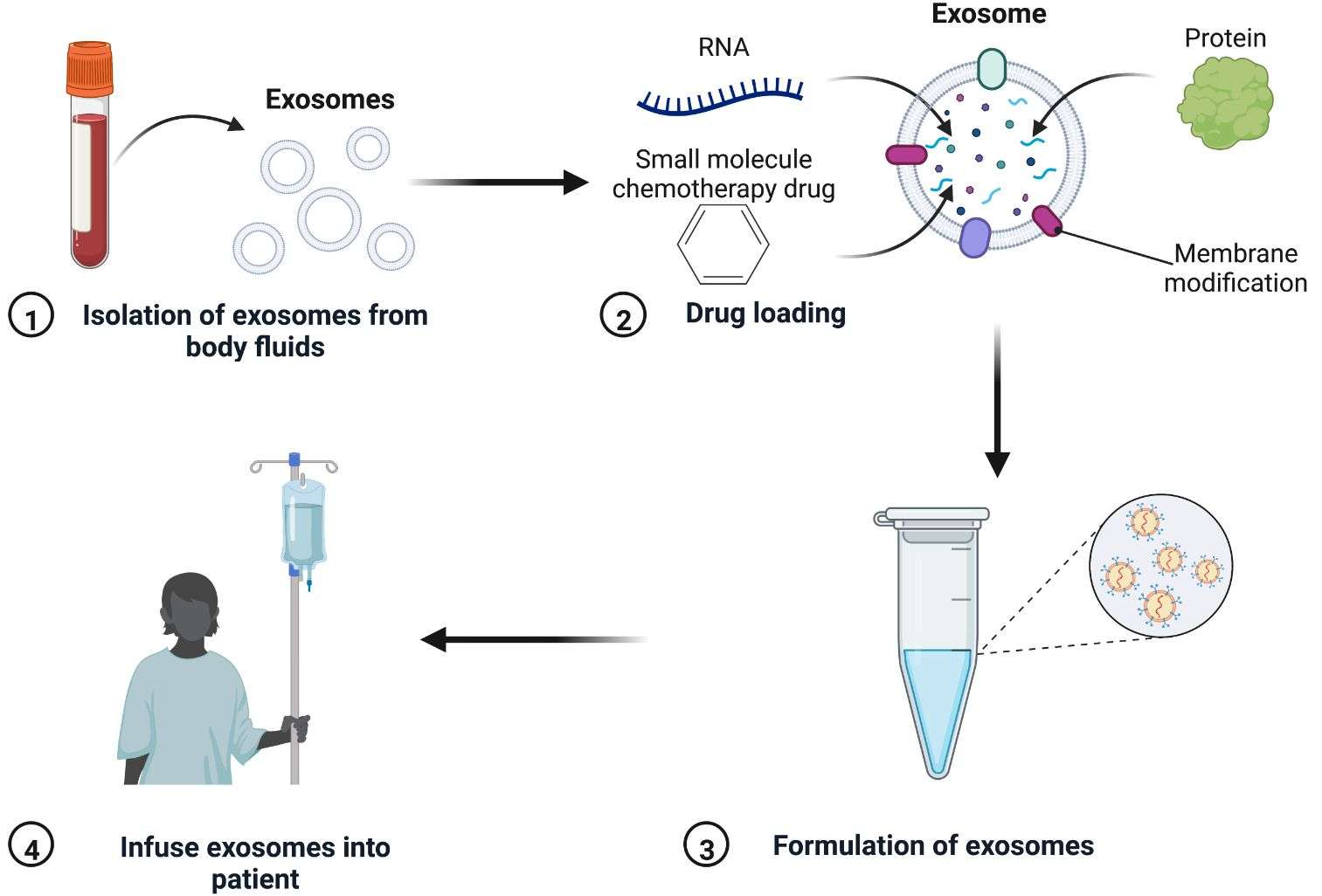 Figure 1. Process flow of drug-loaded exosomes. (Zhang Y, et al., 2022)
Figure 1. Process flow of drug-loaded exosomes. (Zhang Y, et al., 2022)
What is Preloading Technology?
Preloading is an engineered loading method based on parental cells. Drugs are loaded into donor cells before exosome isolation, thereby encapsulating them in exosomes during the drug's natural biogenesis. Preloading methods include modifying donor cells to produce targeted exosomes by co-incubating cells with cargo or transfecting cells with target genes. These modified cells are designed to produce and release exosomes containing specific cargoes, such as drugs, proteins, and nucleic acids. Of these, loading proteins, siRNAs, miRNAs, and high molecular weight drugs is advantageous. In addition, preloading methods allow continuous production of cargo-loaded exosomes without damaging membrane integrity.
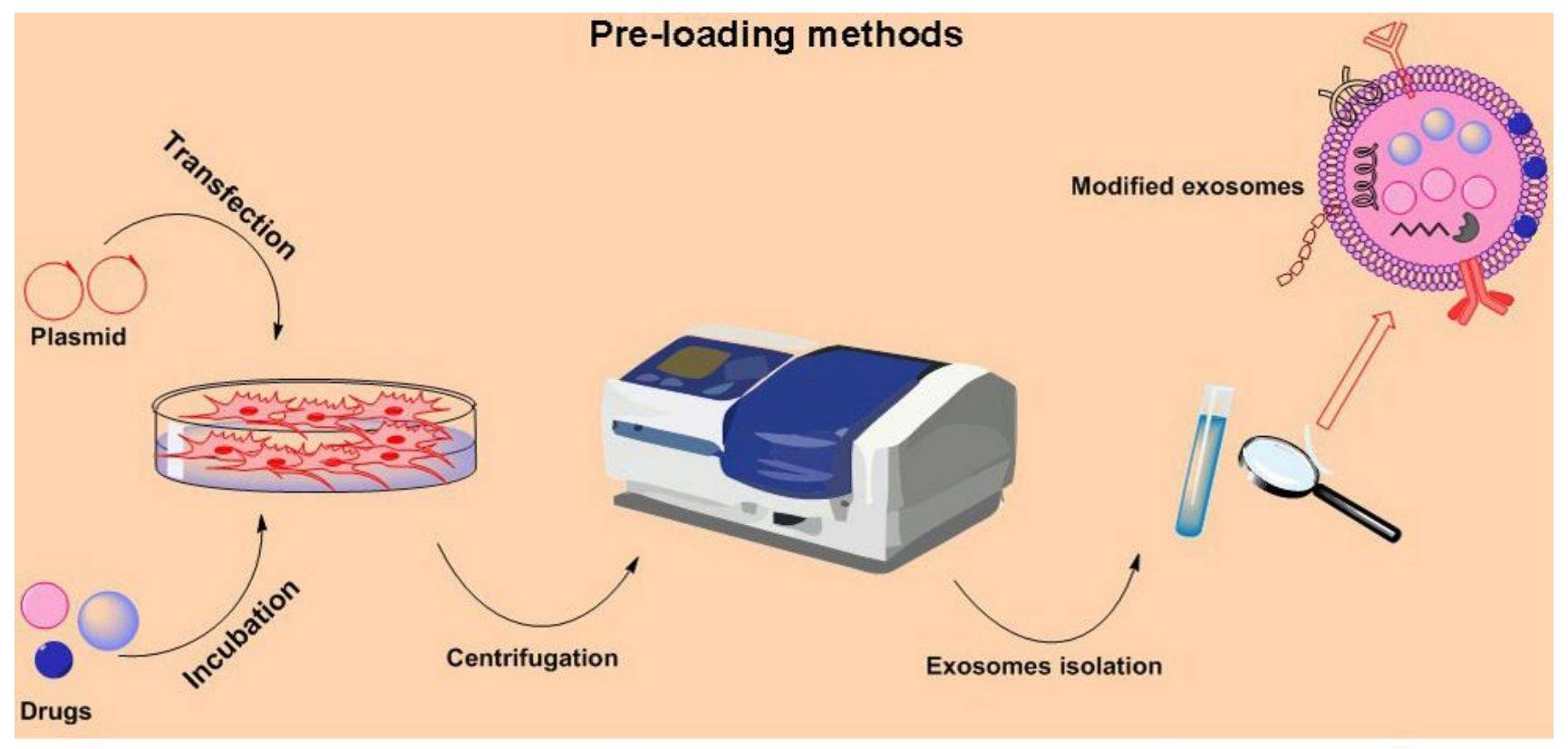 Figure 2. Preloading methods for exosome loading drugs. (Akbari A, et al., 2023)
Figure 2. Preloading methods for exosome loading drugs. (Akbari A, et al., 2023)
- Co- incubation ( Drugs and Cells)
Co-incubation is the co-culture of drugs with cells, where the drug enters the cells and is secreted with exosomes. These exosomes can be isolated from the co-culture supernatant using ultracentrifugation. Although this method is relatively simple, it is limited by relatively low loading efficiency and the inability to precisely control the amount of cargo. Admixture efficiency may be affected by the physicochemical nature of the drug and the type of donor cells used.
- Transfection
Transfection is the most common way to package active molecules such as peptides, proteins, and nucleic acids into exosomes. Introduction of the target gene into the cell, followed by packaging of the gene into the exosome. A variety of techniques have been employed to accomplish this, including the introduction of plasmids, miRNAs, or other nucleic acids into cells. Although transfection ensures that the drug is loaded into the exosome before it is secreted out of the cell, the loading efficiency is low since the loading process is not controlled.
Why Choose Preloading for Exosome Loading?
Stability - Drugs loaded into exosomes using preloading exhibit high stability and are suitable for downstream animal experiments.
Simplicity and scalability - Compared to other drug loading techniques, preloading is simpler to operate and easier to scale up to larger production runs.
Reduced exosome damage - Since preloading typically involves incorporating drugs during exosome biogenesis, it safeguards the exosome structure from damage.
Examples of Exosome Drug Loading by Preloading
Circular RNA (circRNA) is involved in the pathology of major depressive disorder (MDD). Targeted delivery of circRNA to alleviate MDD without the restriction of the blood-brain barrier (BBB) is a promising antidepressant treatment. Researchers co-transfected circDYM-GFP lentivirus and RVG-Lamp2b plasmid with HEK-293T cells and obtained circDYM-containing exosomes (RVG-circDYM-EVs) by differential centrifugation. The results showed that the administration of RVG-circDYM-EVs effectively delivered circDYM to the brain and attenuated CUS-induced depressive-like behavior.
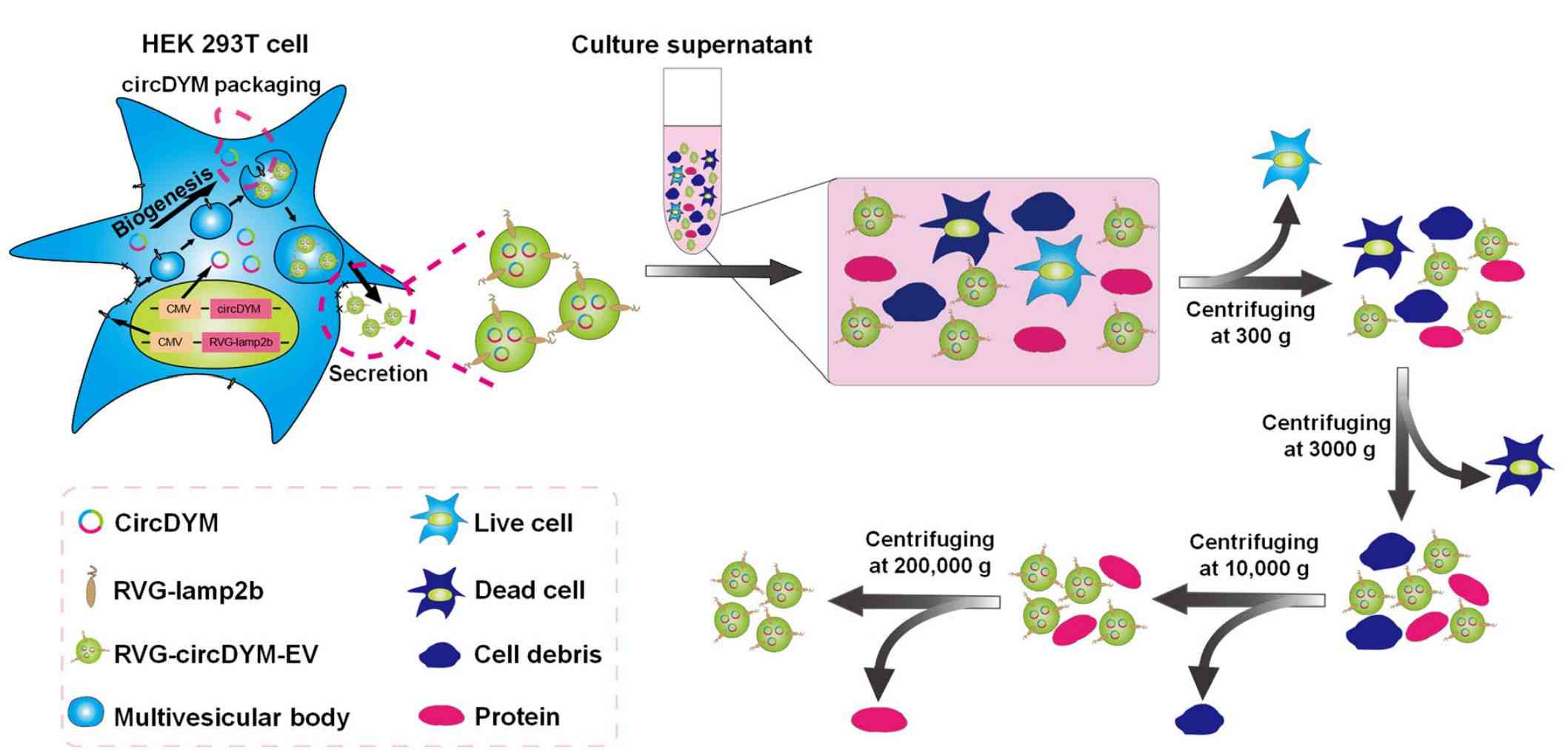 Figure 3. Schematic diagram of the production and harvest of engineered RVG-EVs for targeted circDYM delivery. (Yu X, et al., 2022)
Figure 3. Schematic diagram of the production and harvest of engineered RVG-EVs for targeted circDYM delivery. (Yu X, et al., 2022)
Extracellular vesicles (EVs) are suitable for inducing complex multicellular processes required for wound repair, such as angiogenesis. In recent years, researchers have utilized the ability of EVs to be modified through producer cells to research the potential of mesenchymal stem cells/stromal cells (MSCs) transfected with EVs overexpressing the long non-coding RNA HOX transcriptional antisense RNA (HOTAIR). The results showed that MSCs overexpressing HOTAIR (HOTAIR-MSC) produced EVs with high HOTAIR content that promoted angiogenesis and wound healing in diabetic (db/db) mice.
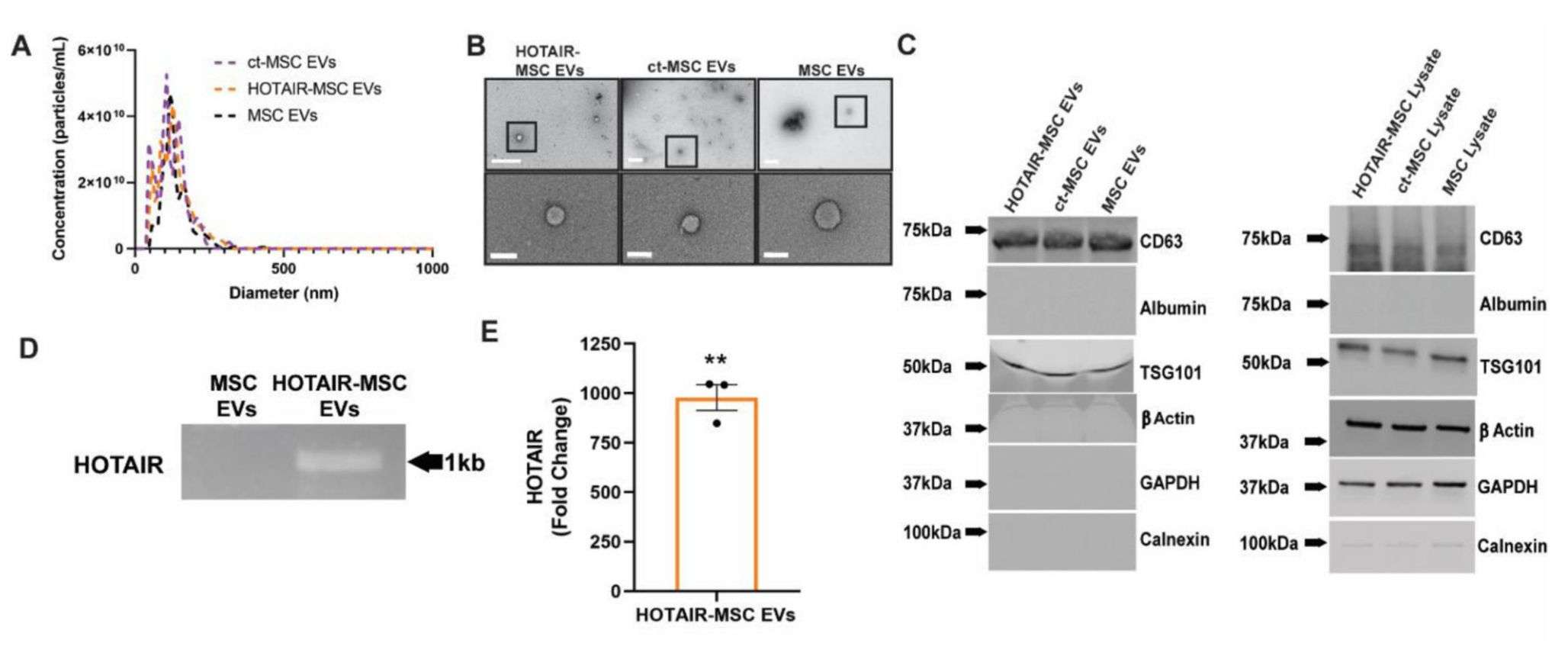 Figure 4. EV characterization and HOTAIR loading confirmation. (Born LJ, et al., 2022)
Figure 4. EV characterization and HOTAIR loading confirmation. (Born LJ, et al., 2022)
Our Products
Exosomes can transfer encapsulated proteins, genetic material, and metabolites to recipient cells and act as information transmitters. Based on the characteristics of exosomes, Creative Biostructure has successfully established exosome drug loading strategies, which can effectively load exogenous cargoes into exosomes.
In addition, we have a rich inventory of exosome products, which can help clients accelerate the progress of exosome projects.
| Cat No. | Product Name | Source |
| Exo-CH15 | HQExo™ Exosome-BLCL21 | Exosome derived from EBV transformed lymphoblastoid B cells (BLCL21 cell line) |
| Exo-CH17 | HQExo™ Exosome-HT29 | Exosome derived from human adenocarcinoma (HT29 cell line) |
| Exo-CH18 | HQExo™ Exosome-LnCAP | Exosome derived from human prostate adenocarcinoma (LnCAP cell line) |
| Exo-HDBF-01 | HQExo™ Exosome-SDH-Alzheimer's plasma | Exosome derived from Single Donor Human Alzheimer's plasma |
| Exo-HDBF-02 | HQExo™ Exosome-SDH-Asthma plasma | Exosome derived from Single Donor Human Asthma plasma |
| Exo-HDBF-03 | HQExo™ Exosome-SDH-Atopic Dermatitis plasma | Exosome derived from Single Donor Human Atopic Dermatitis plasma |
| Exo-IC01 | HQExo™ Exosome-BC3 | Exosome derived from human B lymphocyte cell line (BC-3 ) |
| Exo-IC02 | HQExo™ Exosome-JM1 | Exosome derived from human T pre-B lymphoblast cell line (JM1) |
| Exo-IC03 | HQExo™ Exosome-Jurkat E6-1 | Exosome derived from human T lymphocyte cell line (Jurkat E6-1) |
| PNE-FLB67 | PNExo™ Exosome-Bamboo | Exosome derived from Bamboo |
| PNE-FA95 | PNExo™ Exosome-Annona Squamosa | Exosome derived from Annona Squamosa |
| Explore All Exosomes Products | ||
Creative Biostructure is dedicated to providing efficient exosome services and high-quality exosome products. Our team of scientists has many years of experience in exosome research and is skilled in exosome isolation, characterization, and downstream functional analysis. If you are interested in our services and products, contact us to discuss your project requirements.
References
- Zhang Y, et al. Exosomes as Anticancer Drug Delivery Vehicles: Prospects and Challenges. Front Biosci (Landmark Ed). 2022. 27(10): 293.
- Akbari A, et al. Engineered Exosomes for Tumor-Targeted Drug Delivery: A Focus on Genetic and Chemical Functionalization. Pharmaceutics. 2023. 15(1): 66.
- Yu X, et al. Extracellular vesicle-mediated delivery of circDYM alleviates CUS-induced depressive-like behaviors. J Extracell Vesicles. 2022. 11(1): e12185.
- Born LJ, et al. HOTAIR-Loaded Mesenchymal Stem/Stromal Cell Extracellular Vesicles Enhance Angiogenesis and Wound Healing. Adv Healthc Mater. 2022. 11(5): e2002070.
- Zeng H, et al. Current Strategies for Exosome Cargo Loading and Targeting Delivery. Cells. 2023. 12(10): 1416.
