Ion Exchange Chromatography
As the world leading protein product manufacturer, Creative Biostructure is expertized in providing protein purification technical support to help your day to day research based on our magic MagHelix™ Protein Purification platform.
The separation principle of ion exchange chromatography is based on the different net surface charge of molecules to be purified. Molecules that have varying charge properties prefer interact with charged chromatography media in a variety of interaction degrees. Factors such as the overall charge, surface charge distribution, and charge density will greatly affect the seperation. The net surface charge of a molecule depends on its charged groups, possessing different acid ionization constant values (pKa) as the structure and chemical microenvironment changes.

There are four main steps in ion exchange chromatography:
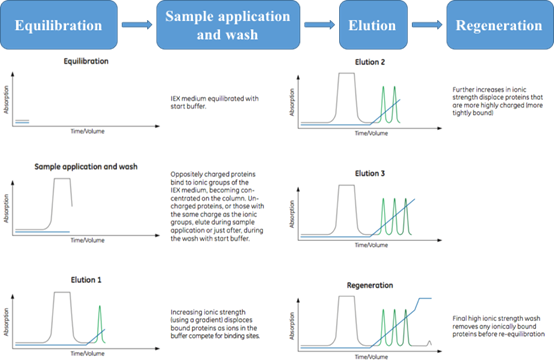 Figure 1. General steps of an anion exchange separation. (Ion Exchange Chromatography Principles and Methods, 11000421)
Figure 1. General steps of an anion exchange separation. (Ion Exchange Chromatography Principles and Methods, 11000421)
- Stationary phase achieved after equilibration, leading to the desired start conditions;
- Sample application and wash step is for target molecule binding and other unbound materials washing out;
- In most cases, target molecules elution are based on the increased ionic strength (salt concentration) of the buffer or the changed pH;
- Regeneration step is crucial for next run, ensuring that the stationary phase maintain the full capacity.
Practical considerations for ion exchange separation:
1. Ionic strength and buffer pH
Ionic strength and buffer pH have to be compatible with stability and activity of target protein.
Should avoid extreme changes that may lead to inactivation or precipitation.
Checking the stability at the selected ionic strength values and pH value in case of considering the recovery of biological activity as the priority.
2. Anion or cation exchanger
Below pI of sample components, most stable, using a cation exchanger.
Above pI of sample components, most stable, using an anion exchanger.
If high stability of sample components can be achieved on both sides of the pI, using either ion exchanger will be OK.
3. Buffer selection and preparation
The buffer concentration typically range from 20 to 50 mM, sufficiently maintaining buffering capacity and constant pH.
Using volatile buffers in case the purified protein is to be lyophilized.
The selected buffers must have appropriate acid ionization constant values for the working temperature as the acid ionization constant values of the buffer varies with temperature.
4. Counterions
In most cases: Na+ for cation exchange, while Cl– for anion exchange.
5. Flow rates
Saving time by applying higher flow rates for the high salt washing process and re-equilibration steps. Keep in mind that do not exceed the maximum flow rate.
Operating pressures increases as flow rates increases.
Good resolution and reproducibility can be achieved by accurate and reproducible flow control.
Our support team is currently developing the Support Centers for Protein Purification service, and other technical resources are listed as below. Please feel free to Contact Us.