Structural Research of Alphaflexiviridae
The Alphaflexiviridae family contains viruses that infect plants and a few viruses discovered in plant-infecting fungi. At present, there are 51 species in this family, divided among 6 genera. Viruses in this family are non-enveloped, with flexuous and filamentous geometries, typically 12-13 nm in diameter and about 470-800 nm in length, depending on the genera. They have helical symmetry with a pitch of about 3.4 nm (range from 3.3 to 3.7 nm) and there are clearly visible cross-bands in some genera. The viruses of this family contain a single molecule of linear ssRNA of about 5.9-9.0 kb in length, which codes for 1-6 proteins. They have a unique alphavirus-like replication protein lineage that is unusual in the absence of any recognized protease domain. The viral capsids of all members of the family (except for Lolavirus) consist of a single polypeptide ranging in size from 18 to 43 kD. Virions are generally highly immunogenic, and within the genus, some viruses are associated with serology. Many viruses have a relatively mild effect on their hosts, and all species can be transmitted by mechanical inoculation.
Plant viruses and their virus-like particles (VLPs) have many advantages for biotechnological applications, the most significant of which is complete safety for humans. Here are two potentially promising objects for the design of novel materials in this family, Alternanthera mosaic virus and Papaya mosaic virus.
Alternanthera mosaic virus
Alternanthera mosaic virus (AltMV) is a new virus isolated from Alternanthera pungens plants of the Amaranthaceae family and was first isolated and described in Australia in 1999. The AltMV genome is a sense single-stranded RNA with a length of 6604-6607 nt. AltMV RNA contains a cap at the 5' end and a poly(A) sequence at the 3' end, encoding five proteins which are 174 kDa viral replicase, three motor proteins (molecular weight 26 kDa, 12 kDa, and 7 kDa) and 22 kDa coat protein. The flexible filamentous virion particles of AltMV have helical symmetry and are composed of coat protein subunits. The average length of AltMV virions is about 570 nm, and the length depends on the isolate. The virions were approximately 13 nm in diameter and the AltMV virion diameter was recently corrected to 13.5 nm by cryo-electron microscopy. AltMV is the closest relative of papaya mosaic virus (PapMV) based on its serological properties and similarity of nucleotide and amino acid sequence.
Recently, the three-dimensional structures of AltMV virions and their VLPs with ~13Å resolution were obtained by single-particle cryo-electron microscopy. The results of the three-dimensional structural reconstruction comparison indicate that the coat protein of AltMV has different folds in the presence of viral RNA (virions) and in the absence of viral RNA (VLP), which has been confirmed by structural analysis by trypsin treatment. Therefore, the structure of morphologically similar virions and virus-like particles based on the coat protein of the filamentous plant virus shows a different structure for the first time. Nonetheless, AltMV virions and VLPs have been shown to be stable under a variety of conditions, including serum from experimental animals. The main difference between the VLP of AltMV and the VLP of the related papaya mosaic virus is the high stability of the AltMV VLP under a wider range of conditions.
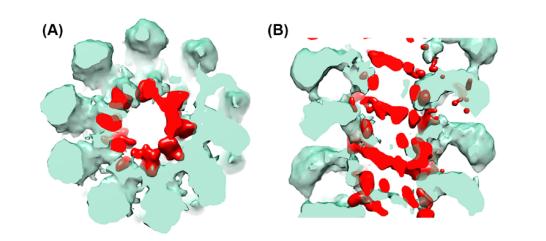 Figure 1. Difference map (red) between aligned to each other AltMV VLP and virion, superimposed onto a 3D structure of a VLP (transparent green) (A) Sagittal section (B) Cross section.
Figure 1. Difference map (red) between aligned to each other AltMV VLP and virion, superimposed onto a 3D structure of a VLP (transparent green) (A) Sagittal section (B) Cross section.
Papaya mosaic virus
Papaya mosaic virus (PapMV) contains a sense single-stranded RNA of 6656 nt in length encoding five proteins. The coat protein is the only structural protein of the virus. It is a peptide containing 215 amino acid residues and self-assembles around the viral RNA to become a 530 nm long flexible helical symmetric capsid. Modified forms of PapMV coat proteins and proteins can be produced in bacteria in which they self-assemble into nanoparticles similar to wild-type PapMV viruses isolated from infected plants. The expression system can be used for biochemical studies of PapMV coat protein, and for the production of chimeric recombinant nanoparticles, which can be used as an adjuvant or vaccine platform. PapMV nanoparticles appear to be recognized by the innate immune system as a molecular pattern associated with pathogens. This property makes it an excellent immunomodulator for improving seasonal influenza or typhoid vaccine candidates.
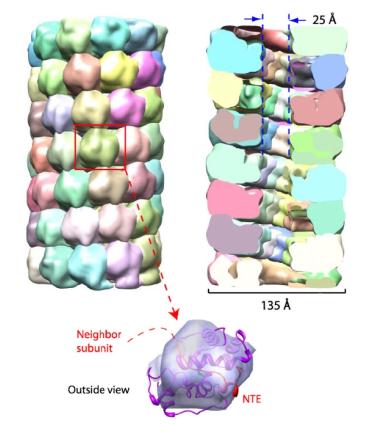 Figure 2. Density segmentation and docking of the cryo-EM map of PapMV.
Figure 2. Density segmentation and docking of the cryo-EM map of PapMV.
Creative Biostructure has been and will always support researchers in finding the best solutions. We provide off-the-shelf VLP products that are ready for immediate worldwide delivery. If our current catalog does not include the VLP products you are looking for, contact our representatives to ask for our custom VLP construction services, which include gene synthesis, protein expression, processing, and packing, and our services can meet your specific requirements on formulations and modifications of your target VLPs. In addition, we focus on structural biology research, providing contract services for viral particle identification and characterization.
References
- Donchenko E K, et al. Structure and properties of virions and virus-like particles derived from the coat protein of Alternanthera mosaic virus. PLoS One. 2017. 12(8): e0183824.
- Donchenko E, et al. Alternanthera mosaic potexvirus: several features, properties, and application. Advances in Virology. 2018.
- Yang S, et al. Crystal structure of the coat protein of the flexible filamentous papaya mosaic virus. Journal of Molecular Biology. 2012. 422(2): 263-273.
