Exosomes Target Delivery Based on Genetic Engineering
Exosomes are cell-secreted external vesicles involved in the intercellular transport of substances and have great therapeutic potential in cancer and chronic diseases due to special properties such as low toxicity, low immunogenicity, and high biocompatibility. Therapeutic agents (e.g., small molecules or nucleic acid drugs) can be integrated into exosomes and then delivered to specific cells or tissues for targeted drug delivery.
However, natural exosomes are poorly targeted, greatly reducing therapeutic efficacy. Therefore, there is a need to utilize engineering technologies to attach targeting units to the surface of the exosome membrane or to fit into the lumen, so that the exosomes can acquire active targeting ability to aggregate in specific cell types and tissues. The most common approach is genetic engineering technology to enable donor cells to overexpress targeting proteins or peptides to secrete target-specific exosomes.
How does Genetic Engineering Enable Exosomes Targeted Delivery?
Genetic engineering techniques manipulate genes at the molecular level by genetically modifying donor cells to express a target protein on the surface of the cell membrane, which in turn is stably displayed on the surface of exosomes secreted by the cell. Research has confirmed that certain proteins can interact with cellular receptors or extracellular matrix components expressed in cells of specific systems. To confer stronger targeting properties to exosomes, genes expressing these targeting proteins or polypeptides are transferred into donor cells, which then secrete exosomes carrying specific homing proteins or polypeptides. This method utilizes the inherent protein expression mechanism of the cell and the natural biosynthesis of exosomes, thereby facilitating the maintenance of the natural conformation and function of the expressed target proteins or polypeptides.
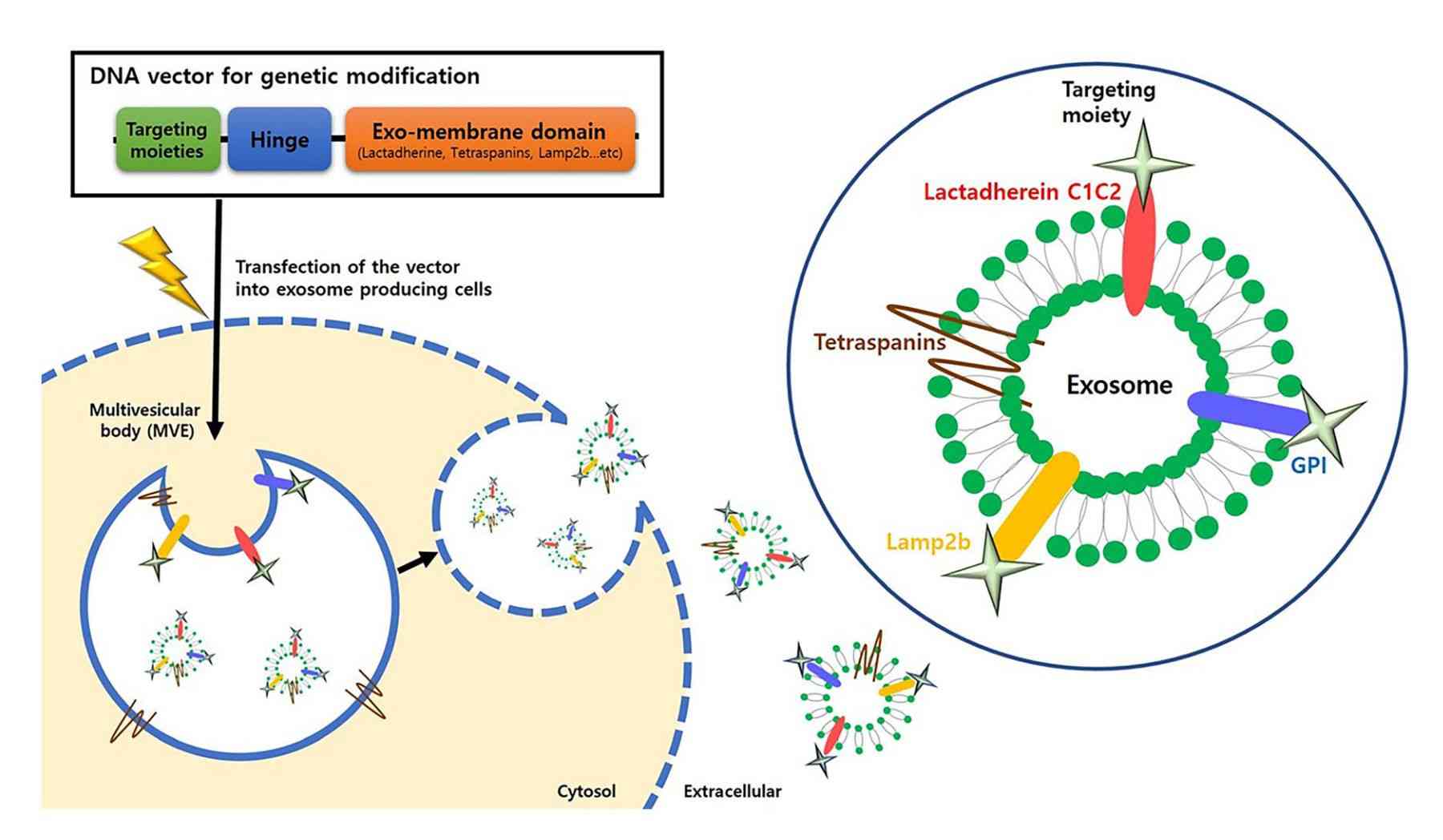 Figure 1. Schematic of genetic modification strategies for active targeting of exosomes. (Choi H, et al., 2021)
Figure 1. Schematic of genetic modification strategies for active targeting of exosomes. (Choi H, et al., 2021)
Common Exosome Anchor Targeting Sites
- LAMP-2B is the most widely used exosome surface protein displaying targeting motifs and belongs to the lysosome-associated membrane protein (LAMP) family. LAMP-2B protein has been reported to be abundantly expressed on exosomes of dendritic cell origin. It is demonstrated that the N-terminus of LAMP-2B is displayed on the exosome surface and can be appended with targeting sequences. It is possible to screen cell-specific binding peptides for specific organs or tissues by phage display and genetically modify the N-terminus of LAMP-2B for its targeting.
- In addition to LAMP-2B, the transmembrane protein platelet-derived growth factor receptor (PDGFR) is commonly used for membrane display. For example, the fusion of the GE11 gene to the PDGFR transmembrane region using the pDisplay vector produces GE11 exosomes in 293T cells. GE11 exosomes exhibit high affinity and low mitogenic activity for epidermal growth factor receptor (EGFR)-overexpressing cancer cells, making them a promising delivery system for EGFR-targeted therapies.
- The four-transmembrane protein superfamily CD63/CD9/CD81 has two extracellular loops that can also be designed to display targeting sequences or probes. To develop a therapeutic approach for Duchenne muscular dystrophy (DMD), the fusion of the inhibitory structural domain of the muscle growth inhibitory pre-peptide to the second extracellular loop of CD63 significantly improved its serum stability and delivery efficiency. Injection of exosomes of the pre-peptide of muscle growth inhibitor promotes muscle repair and proliferation, thereby significantly blocking muscle degeneration.
Advances in Targeted Delivery of Genetically Engineered Modified Exosomes
Targeted delivery to diseased cells or tissues is critical for the successful clinical application of nucleic acid drugs. In particular, delivery of microRNA-140 (miR-140) to chondrocytes remains difficult. Therefore, by fusing chondrocyte affinity peptide (CAP) with Lamp2b on the surface of exosomes, researchers obtained CAP-exosomes, which can efficiently encapsulate miR-140, specifically enter and deliver the cargo into chondrocytes. CAP-exosomes are retained in the joint after intra-articular injection and deliver miR-140 to deep cartilage regions via mesochondrium to inhibit cartilage-degrading proteases. The results suggest that targeted delivery by chondrocyte-targeted exosomes as miR-140 carriers is a new therapeutic approach for osteoarthritis (OA).
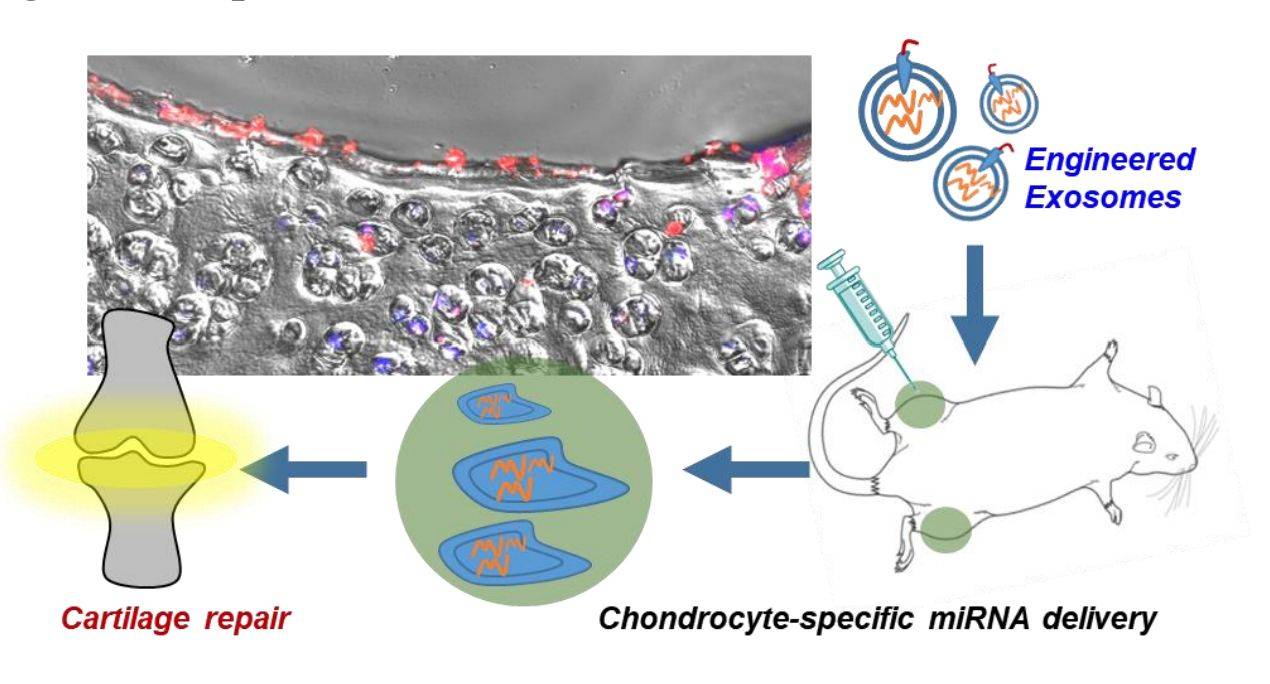 Figure 2. Schematic of engineered exosome-targeted delivery of miRNA for osteoarthritis therapy. (Liang Y, et al., 2020)
Figure 2. Schematic of engineered exosome-targeted delivery of miRNA for osteoarthritis therapy. (Liang Y, et al., 2020)
Multifunctional exosomes with tumor-targeted imaging and therapeutic potential have attracted extensive interest in cancer research. Researchers developed a multifunctional targeted delivery platform based on HEK-293T exosomes by modifying HEK-293T cells to express exosome membrane proteins fused to αv integrin-specific iRGD peptides and tyrosine fragments (Lamp2b). This platform is loaded with adriamycin (Dox) and labeled exosomes using radioactive 131I. Confocal imaging and flow cytometry experiments demonstrated that iRGD exosomes efficiently targeted and delivered Dox to integrin αvβ3-positive interstitial thyroid cancer (ATC) cells. In vivo labeling of exosomes with 131I confirmed the excellent tumor-targeting ability of iRGD exosomes by imaging devices.
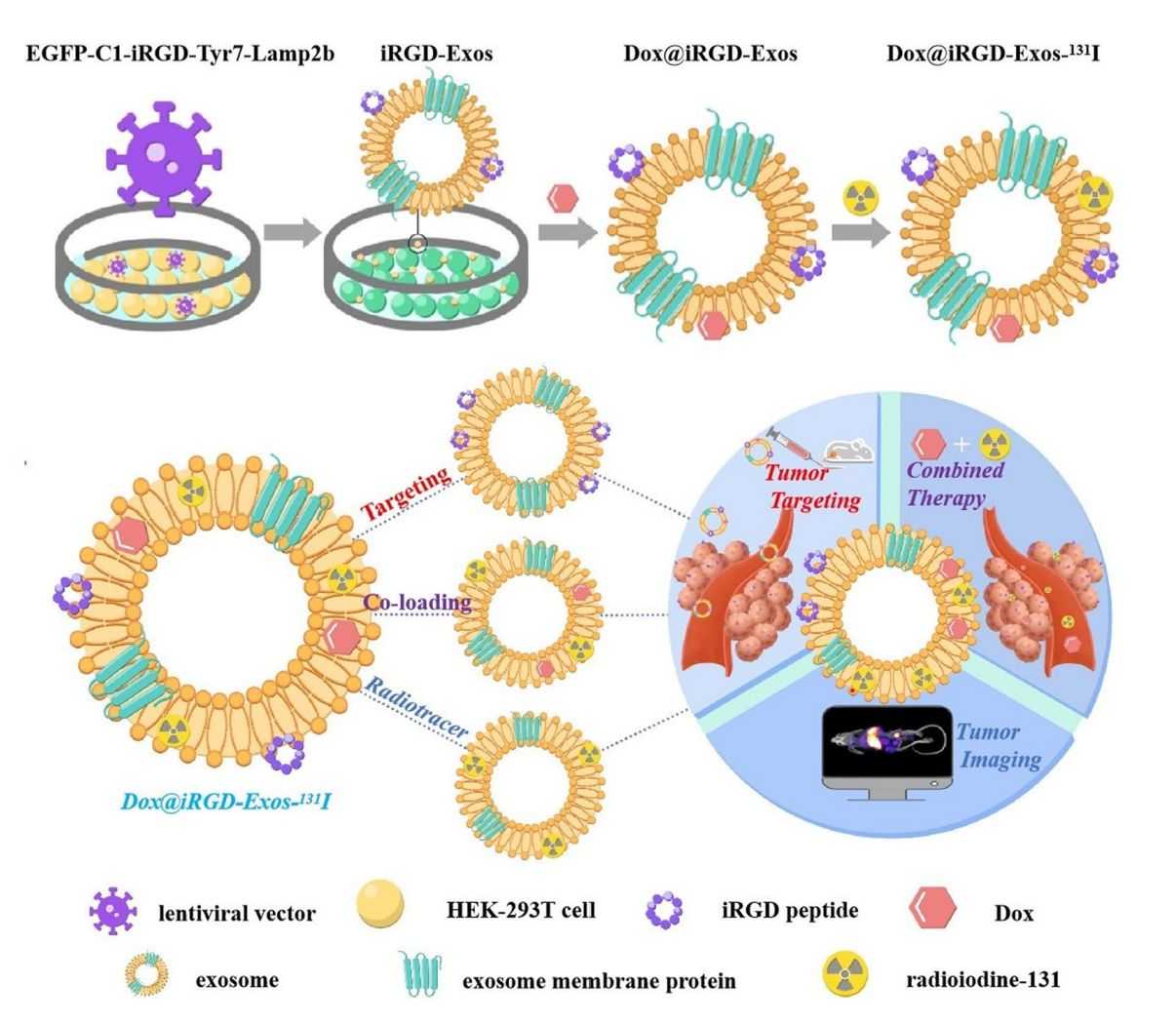 Figure 3. HEK-293T exosome-targeted delivery platform for targeted tumor chemotherapy. (Wang C, et al., 2022)
Figure 3. HEK-293T exosome-targeted delivery platform for targeted tumor chemotherapy. (Wang C, et al., 2022)
Our Services and Products
Although unmodified natural exosomes have targeting ability, they still suffer from low targeting efficiency, short circulating half-life, and poor therapeutic efficacy. Using various engineering methods, the homing peptide or ligand on the surface of exosomes can be modified to confer exosomes with targeting ability, thus improving therapeutic efficiency.
Creative Biostructure, as an expert in exosome research, provides clients with aptamer-based exosome targeting services. We also offer a comprehensive range of exosome products that can be used in genetic engineering-based exosome targeting modification research.
| Cat No. | Product Name | Source |
| Exo-CH05 | HQExo™ Exosome-H841 | Exosome derived from human small cell lung cancer cell line (H841 cell line) |
| Exo-CH17 | HQExo™ Exosome-HT29 | Exosome derived from human adenocarcinoma (HT29 cell line) |
| Exo-HDBF-02 | HQExo™ Exosome-SDH-Asthma plasma | Exosome derived from Single Donor Human Asthma plasma |
| Exo-IC01 | HQExo™ Exosome-BC3 | Exosome derived from human B lymphocyte cell line (BC-3 ) |
| Exo-IC02 | HQExo™ Exosome-JM1 | Exosome derived from human T pre-B lymphoblast cell line (JM1) |
| Exo-SC03 | HQExo™ Exosome-hTERT | Exosome derived from hTERT-immortalized Mesenchymal Stem Cell |
| Exo-SC04 | HQExo™ Exosome-MSC | Exosome derived from Xeno-Free Human Mesenchymal Stem/Stromal Cells and Media |
| Exo-IC01 | HQExo™ Exosome-BC3 | Exosome derived from human B lymphocyte cell line (BC-3 ) |
| Exo-GC07 | HQExo™ Exosome-CD63-EGFP | Exosome derived from human embryonic kidney cell line (HEK293, CD63-EGFP) |
| PNE-FLA16 | PNExo™ Exosome-Aloe | Exosome derived from Aloe |
| PNE-VA10 | PNExo™ Exosome-Asparagus | Exosome derived from Asparagus |
| Explore All Exosomes Products | ||
We have many years of experience in successful projects to provide our clients with high-quality and innovative exosome targeting delivery solutions. If you are interested in our services and products, please feel free to contact us for a detailed quotation.
References
- Choi H, et al. Biodistribution of Exosomes and Engineering Strategies for Targeted Delivery of Therapeutic Exosomes. Tissue Eng Regen Med. 2021. 18(4): 499-511.
- Liang Y, et al. Chondrocyte-Targeted MicroRNA Delivery by Engineered Exosomes toward a Cell-Free Osteoarthritis Therapy. ACS Appl Mater Interfaces. 2020. 12(33): 36938-36947.
- Wang C, et al. Engineering a HEK-293T exosome-based delivery platform for efficient tumor-targeting chemotherapy/internal irradiation combination therapy. J Nanobiotechnology. 2022. 20(1): 247.
- Xu M, et al. Engineered exosomes: desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics. 2021. 11(18): 8926-8944.
- Yang Q, et al. Exosome-based delivery strategies for tumor therapy: an update on modification, loading, and clinical application. J Nanobiotechnology. 2024. 22(1): 41.