Structural Research of Chain Length Determinant and Associated Proteins
Lipopolysaccharides (LPSs) represent an indubitable and fundamental component of the outer membrane of Gram-negative bacteria, playing a multifaceted and intricate role in an assortment of biological processes, ranging from cell recognition, host-pathogen interactions, and virulence. The structure of LPSs is determined by a series of enzymes, including the chain-length determinant proteins, which are responsible for controlling the length of the O-antigen polysaccharide chain. Without these proteins, the LPS synthesis process would be impaired and potentially catastrophic.
The Wzy-dependent pathway of polysaccharide biosynthesis, which is implicated in the synthesis of these LPSs, is an extraordinarily important and intricate process that warrants closer examination and exploration. Recent advances in the field of single-particle cryo-electron microscopy have enabled the elucidation and resolution of the structure of WzzB, which is one of the co-polymerase components of this pathway. The structure of WzzB, resembling a box jellyfish, consists of an octameric assembly, including a large bell-shaped periplasmic domain. The 2-helix transmembrane domain of each protomer serves to encircle and embrace a vast and seemingly empty transmembrane chamber, which further underscores the complexity and intricacy of this process. Through this structure, the intricate and elaborate architecture of the transmembrane domain is also revealed, including the location of critical and essential residues that are pertinent and central to the Wzz-family of proteins.
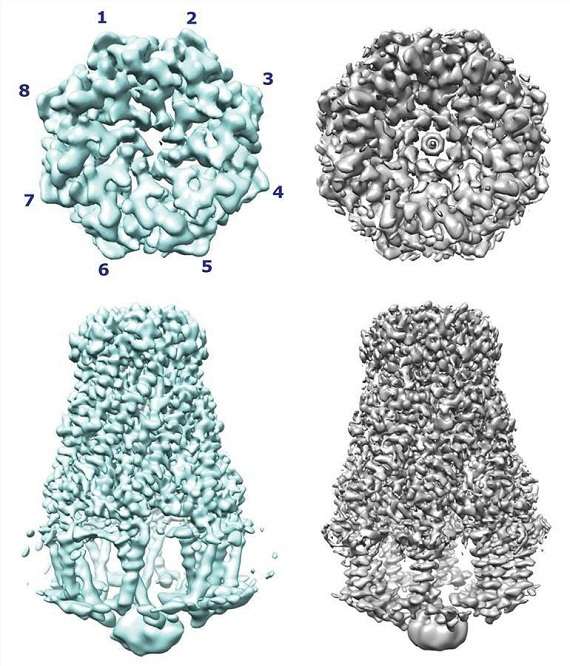 Figure 1. The overall structure of WzzB. (Wiseman B, et al., 2021)
Figure 1. The overall structure of WzzB. (Wiseman B, et al., 2021)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Oligomeric Wzz protein (expressed in E. coli) | Staphylococcus aureus | Cryo-EM single particle analysis | 9.00 Å | 5NBZ |
| WzzB co-polymerase component of the Wzy-dependent pathway (expressed in E. coli) | Escherichia coli | Cryo-EM single particle analysis | 3.00 Å | 6RBG |
| Wzc capsule assembly protein, K540M C1 (expressed in E. coli) | Escherichia coli | Cryo-EM single particle analysis | 2.85 Å | 7NHR |
Table 1. Structural research of chain-length determinant protein involved in lipopolysaccharide synthesis.
Are you on the lookout for a reliable partner to fulfill and cater to your structural research needs? Look no further than Creative Biostructure, which stands out as the epitome of excellence and expertise in this field. Our crack team of specialists boasts of an unparalleled and unsurpassed mastery of cutting-edge and state-of-the-art techniques like cryo-EM, X-ray crystallography, and NMR spectroscopy, which enables and empowers us to gain a profound and comprehensive understanding of the intricate and complex structure and function of membrane proteins.
Our clients can take full advantage and leverage the benefits of our comprehensive and all-encompassing structural analysis service, which spans from protein expression and purification to the ultimate and final structure determination. We pride ourselves on delivering and producing high-quality, accurate, and prompt structural analysis results that are second to none, which sets us apart and distinguishes us from the rest of the competition. If you are looking to delve and plunge into the intricate and enigmatic world of structural research of the chain length determinant and associated proteins, then don't hesitate to contact us. Our team of experts is always ready and eager to discuss and explore your research needs, no matter how challenging or demanding they may seem and offer the best possible and optimal solutions and strategies for your project.
References
- Collins R F, et al. Full-length, oligomeric structure of Wzz determined by cryoelectron microscopy reveals insights into membrane-bound states. Structure. 2017, 25(5): 806-815. e3.
- Wiseman B, et al. Structure of a full-length bacterial polysaccharide co-polymerase. Nature Communications. 2021, 12(1): 369.
- Yang Y, et al. The molecular basis of regulation of bacterial capsule assembly by Wzc. Nature Communications. 2021, 12(1): 4349.