Labeling Exosomes with Radioisotopes
Exosomes carry cargoes of proteins, lipids, mRNAs, and other biologically active substances. Research has confirmed that exosomes play a critical role in cell-to-cell communication, controlling many physiological processes and diseases, including inflammation, cancer, neurological, cardiovascular, and autoimmune diseases.
In the last decade, exosomes from different biological sources have been proposed as new natural platforms for drug delivery. Translating these nanotools into clinical practice requires an in-depth understanding of their pharmacokinetic properties and biodistribution. In recent years, the application of radioisotope labeling techniques in exosome tracking in vivo has received increasing attention.
What is Radioisotope Labeling?
Radioisotope labeling is a classical laboratory labeling technique that has been developed and applied in exosome tracking research. Compared to conventional optical labeling techniques, radiolabeling offers high sensitivity and stable imaging performance in tissue imaging, in vivo imaging, and even clinical imaging. These signals can be detected non-invasively by single photon emission computed tomography (SPECT) or positron emission tomography (PET). Isolated organs can also be imaged by computed tomography (CT) or magnetic resonance (MR). High-resolution anatomical detail information provides a more comprehensive understanding of exocrine dynamics.
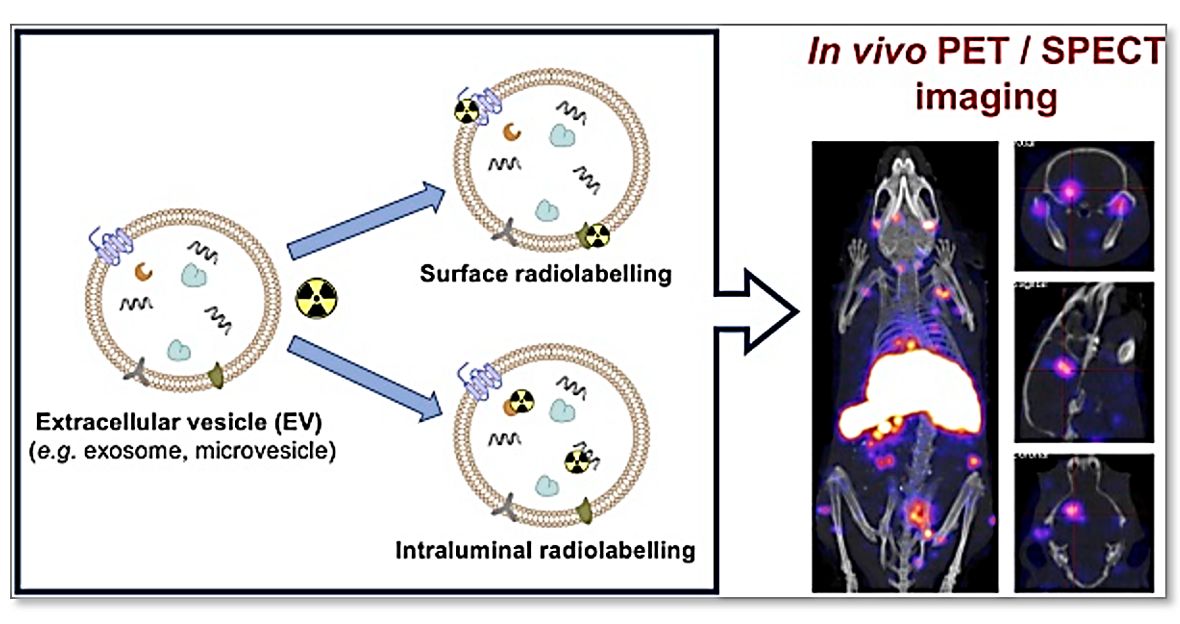 Figure 1. Schematic diagram for labeling exosomes with radioisotopes. (Khan AA, T M de Rosales R., 2021).
Figure 1. Schematic diagram for labeling exosomes with radioisotopes. (Khan AA, T M de Rosales R., 2021).
What are the Commonly used Radioisotopes for Labeling Exosomes?
PET imaging is based on positron-emitting radionuclides. Radionuclides commonly used to label exosomes include iodine-123, technetium-99m, and indium-111.The positrons emitted by these radioisotopes annihilate nearby electrons, thus emitting a pair of gamma rays of comparable energy in opposite directions. The gamma rays are then detected by a ring of detectors that map the incoming radiation. Zirconium-89, copper-64, gallium-68, and fluorine-18 are radionuclides commonly used in PET imaging, with a resolution about two to three times that of SPECT.
What are the Types of Radiolabeling of Exosomes?
- Surface Radiolabeling
The most popular technique for exosome radiolabeling is surface or membrane radiolabeling. Radionuclides are directly or indirectly matched to proteins of the membrane by forming covalent chemical bonds. The four mechanisms of radioisotope attachment include
1. Genetic alignment.
2. Direct cementation of radionuclides from membrane proteins.
3. Direct integration into membrane proteins.
4. Adhesion of radionuclides to surfaces via chelating agents.
Radionuclide assimilation, which involves interactions between radionuclides and exosomal membrane elements. In conventional bioconjugate chemistry, radiotracers are usually attached to membranes by surface chelation methods using surface amine groups. Numerous studies have shown the essential nature of proteins and lipids on the surface of exosomes in determining their behavior. Thus, it is possible to significantly alter those proteins that allow radiolabeling to better target tissues or understand exosome function.
- Intravascular Radiolabeling
An alternative approach to radiolabeling exosomes is to trap the radiotracer in the intravascular space. Because the radionuclide is exposed during epitope labeling, there is the potential for additional exosome counterchelation. For intraluminal radiolabeling of exosomes, the radionuclide must cross the lipid bilayer, and both ion carrier-chelator binding and remote loading can accomplish this. Ion carrier-chelator binding methods utilize popular ion carrier ligands mixed with the radioactive metal to form a substrate, which in turn forms a neutral complex that allows them to be transported across the lipid membrane.
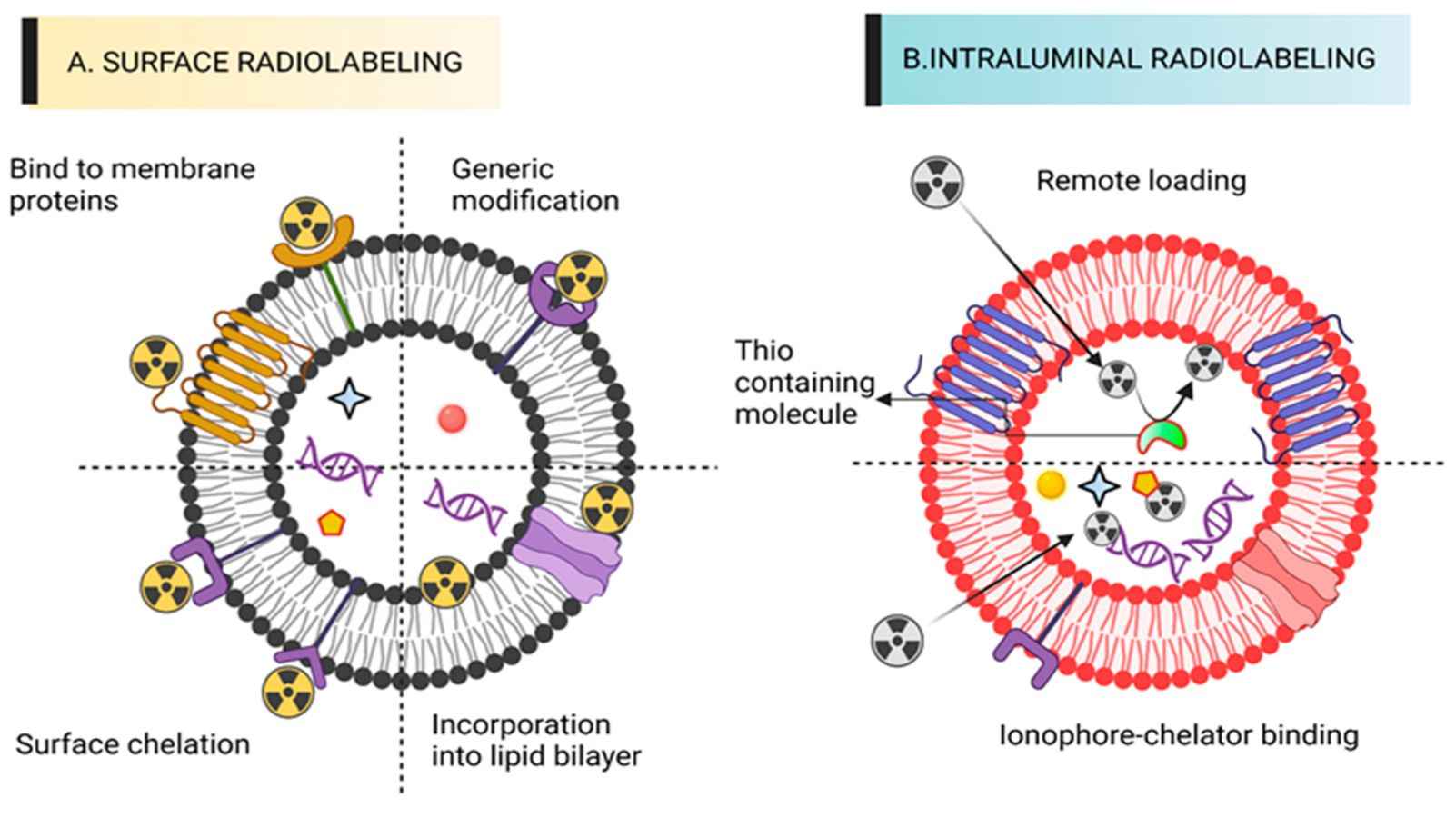 Figure 2. Schematic of exosome surface radiolabeling and luminal radiolabeling. (Ashique S, Anand K., 2023).
Figure 2. Schematic of exosome surface radiolabeling and luminal radiolabeling. (Ashique S, Anand K., 2023).
Advances in Research on Radioisotope Labeling of Exosomes
Iodine radioisotopes are a class of isotopes widely used for labeling. Researchers have shown the distribution of radioisotope 131I-labeled tumor exosomes from different cellular sources in vivo by SPECT/CT. By combining isotope labeling with genetic engineering techniques, streptavidin-lactamyxin fusion proteins, and 125I-labeled biotins are used to form 125I-labeled exosomes. In addition, researchers have directly labeled exosomes with [124I]NaI and monitored them by PET, demonstrating that exosomes accumulate rapidly in the liver and are present in several organs, including the brain.
Metal isotopes have also been widely used. Two techniques for labeling exosomes based on 111In are reported. One is end-labeling, in which the isotope mediated by tropinone crosses the membrane into the lumen of the exosome. The other is a membrane labeling technique based on the bidirectional binding of amine-responsive DTPA-anhydride to the exosome membrane and the isotope. In contrast, membrane labeling is more effective and flexible.99mTc is the most used isotope in the field of exosome labeling. An ionic salt (99mTcCl4) labeling method based on 99mTc is reported. Compared with previous complex compound labeling such as 99mTc-HMPAO, 99mTc-tricarbonyl, and [ 99mTc(CO)3(H2O)3]+, the ionic salt labeling is simpler to operate and less costly and also ensures labeling efficiency.
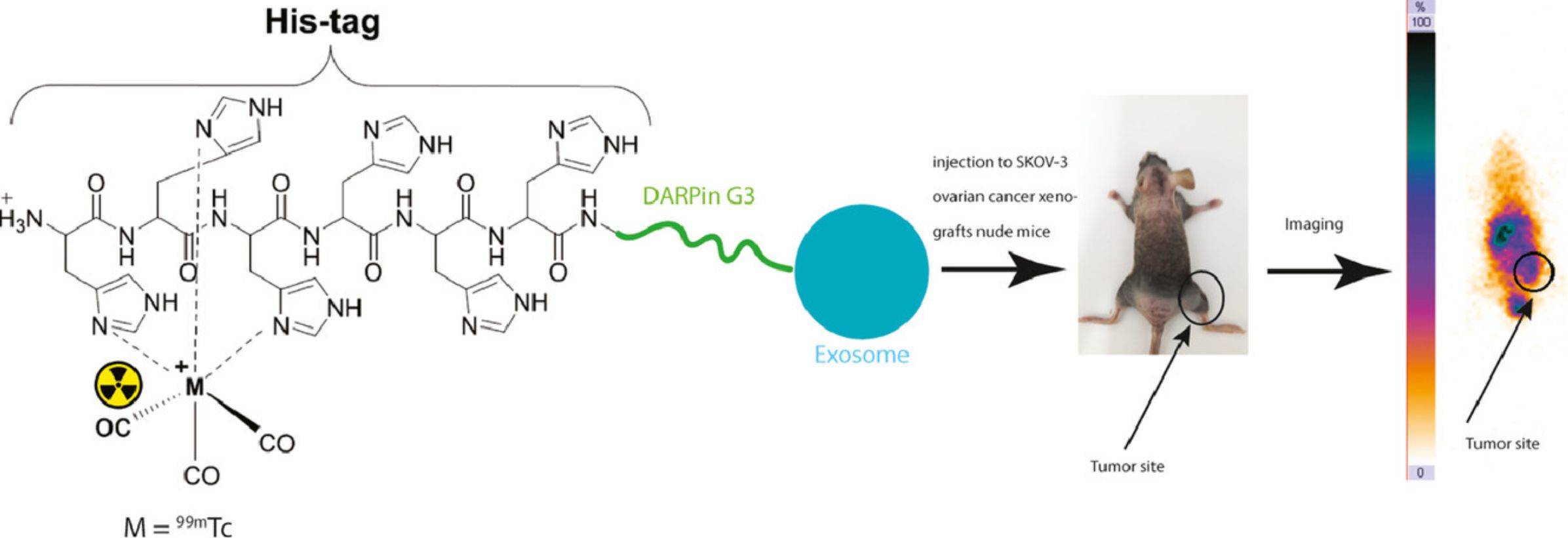 Figure 3. 99mTc-radiolabeled HER2 targeted exosome for tumor imaging. (Molavipordanjani S, et al., 2020).
Figure 3. 99mTc-radiolabeled HER2 targeted exosome for tumor imaging. (Molavipordanjani S, et al., 2020).
Recently, 89Zr-based isotope labeling tracer methods for exosomes have also been reported, including direct labeling of exosomes by amine reaction 89Zr and internal labeling with [89Zr]Zr(oxinate)4. In addition, researchers have successfully visualized the biodistribution of exosomes in lymphatic or blood flow pathways using 68Ga or 64Cu labeling and demonstrated that isotope labeling has higher sensitivity than Cy7 fluorescent labeling. The researchers also performed pharmacokinetic research by labeling exosomes with 64Cu via a cross-bridge macrocyclic chelator (CB-TE1A1P) and determined the contribution of macrophages in the uptake of exosomes by the liver and spleen. In addition, an in vitro exosome molecular imaging technique based on Raman metabolic labeling is reported. Deuterium-labeled exosomes are formed by the cell's metabolism and are used to subsequently track cellular uptake for internalization and other research.
We offer a range of high-quality exosomes isolated from human disease-state body fluids.
| Cat No. | Product Name | Source |
| Exo-HDBF-01 | HQExo™ Exosome-SDH-Alzheimer's plasma | Exosome derived from Single Donor Human Alzheimer's plasma |
| Exo-HDBF-02 | HQExo™ Exosome-SDH-Asthma plasma | Exosome derived from Single Donor Human Asthma plasma |
| Exo-HDBF-03 | HQExo™ Exosome-SDH-Atopic Dermatitis plasma | Exosome derived from Single Donor Human Atopic Dermatitis plasma |
| Exo-HDBF-04 | HQExo™ Exosome-SDH-Benign Breast Conditions plasma | Exosome derived from Single Donor Human Benign Breast Conditions plasma |
| Explore All Exosomes Isolated from Human Disease-state Body Fluids | ||
Why Choose Radioisotopes to Label Exosomes?
- High sensitivity - Radiolabeling allows for high sensitivity, permitting the detection of exosomes at low concentrations.
- Non-invasive - Radiolabeling is non-invasive and does not require the use of antibodies, making it an attractive labeling method for clinical applications.
- Versatile - Radiolabeling can be used for a variety of downstream applications, including imaging, quantification, and tracking of exosomes in vitro and in vivo.
Creative Biostructure has extensive experience in exosome research. If you are interested in our exosome services, please feel free to contact us. We look forward to working with you.
References
- Khan AA, T M de Rosales R. Radiolabelling of Extracellular Vesicles for PET and SPECT imaging. Nanotheranostics. 2021. 5(3): 256-274.
- Ashique S, Anand K. Radiolabelled Extracellular Vesicles as Imaging Modalities for Precise Targeted Drug Delivery. Pharmaceutics. 2023. 15(5): 1426.
- Molavipordanjani S, et al. 99mTc-radiolabeled HER2 targeted exosome for tumor imaging. Eur J Pharm Sci. 2020. 148: 105312.
- Boudna M, et al. Strategies for labeling of exogenous and endogenous extracellular vesicles and their application for in vitro and in vivo functional studies. Cell Commun Signal. 2024. 22(1): 171.
