Structural Research of Adiponectin Receptors
Adiponectin is an antidiabetic adipokine. Human adiponectin receptors 1 and 2 (AdipoR1 and AdipoR2) serve as the major receptors for adiponectin in vivo, belonging to the progestin and adipoQ receptor family1. Adiponectin stimulates its receptors AdipoR1 and AdipoR2, which can then activate the AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor (PPAR) pathways, respectively, to regulate glucose and lipid metabolism, inflammation, and oxidative stress.
Human AdipoR1 and AdipoR2 contain a seven-transmembrane (7TM) helix domain with an internal N-terminus and an external C-terminus, which is the opposite of the topology of G protein-coupled receptors (GPCRs). The crystal structures of human AdipoR1 and AdipoR2 reveal that the 7TM helices form a large internal cavity. In the internal cavity, a zinc ion is coordinated to three conserved histidine residues. In addition, AdipoR1 adopts both the open and closed forms, and their interconversion may be involved in the signaling mechanism.
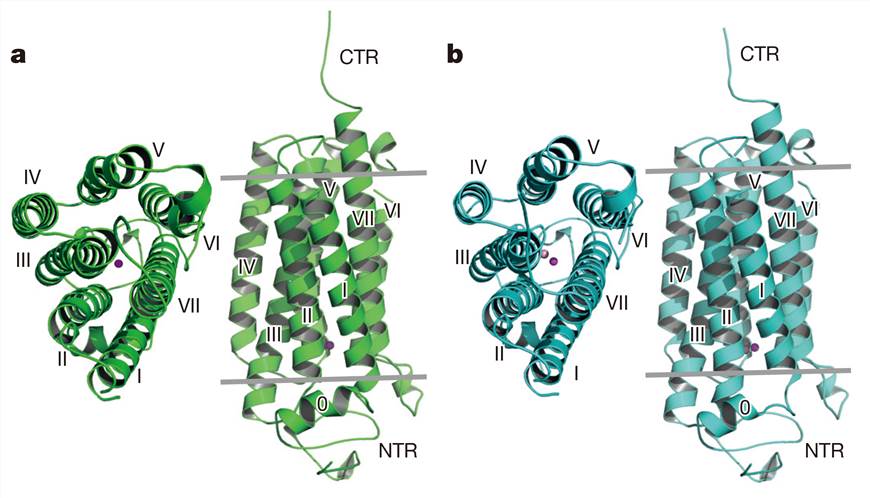 Figure 1. Crystal structures of AdipoR1 and AdipoR2. (Tanabe H, et al, 2015)
Figure 1. Crystal structures of AdipoR1 and AdipoR2. (Tanabe H, et al, 2015)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| AdipoR1 (expressed in Trichoplusia ni, Escherichia coli) | Homo sapiens, Mus musculus | X-ray diffraction | 2.79 Å | 6KS0 |
| AdipoR2 (expressed in Trichoplusia ni, Escherichia coli) | Homo sapiens, Mus musculus | X-ray diffraction | 2.40 Å | 6KS1 |
| AdipoR1 in an open conformation (expressed in Trichoplusia ni, Escherichia coli) | Homo sapiens, Mus musculus | X-ray diffraction | 2.73 Å | 5LXG |
| AdipoR2 in complex with a C18 free fatty acid (expressed in Trichoplusia ni, Drosophila melanogaster) | Homo sapiens, Mus musculus | X-ray diffraction | 2.40 Å | 5LWY |
| AdipoR2 in complex with a C18 free fatty acid (expressed in Spodoptera frugiperda, Drosophila melanogaster) | Homo sapiens, Mus musculus | X-ray diffraction | 2.40 Å | 5LX9 |
| AdipoR2 in complex with a C18 free fatty acid (expressed in Spodoptera frugiperda, Drosophila melanogaster) | Homo sapiens, Mus musculus | X-ray diffraction | 3.00 Å | 5LXA |
| AdipoR1 D208A mutant (expressed in Trichoplusia ni, Escherichia coli) | Homo sapiens, Mus musculus | X-ray diffraction | 3.05 Å | 6KRZ |
| Cryogenic AdipoR2 structure using CrystalDirect (expressed in Drosophila melanogaster) | Homo sapiens | X-ray diffraction | 2.40 Å | 6YX9 |
| Room temperature AdipoR2 structure using CrystalDirect (expressed in Drosophila melanogaster) | Homo sapiens | X-ray diffraction | 2.90 Å | 6YXD |
| Cryogenic AdipoR2 structure with Tb-XO4 ligand using CrystalDirect (expressed in Drosophila melanogaster) | Homo sapiens | X-ray diffraction | 3.01 Å | 6YXG |
| Cryogenic AdipoR2 structure with Gd-DO3 ligand using CrystalDirect (expressed in Drosophila melanogaster) | Homo sapiens | X-ray diffraction | 3.02 Å | 6YXF |
Table 1. Structural Research of Adiponectin Receptors.
X-ray crystallography can obtain the structure of biomolecules with high atomic resolution and is not limited by the molecular weight of the sample. When the X-ray crystallography technique is properly manipulated, it is a powerful tool in providing reliable structural data on biological macromolecules, not only in determining the position and structure of the active center, but also in understanding how protein recognizes and binds ligand molecules at the atomic level.
Creative Biostructure provides X-ray crystallography services backed by our state-of-the-art facilities and has developed a Gene-to-Structure using X-ray crystallography pipeline covering all technical stages from gene synthesis to structure determination. We can offer various crystallization strategies, especially for membrane protein crystallization. If you are interested in our services, please feel free to contact us.
References
- Tanabe H, et al. Crystal structures of the human adiponectin receptors. Nature. 2015, 520(7547): 312-316.
- Vasiliauskaité-Brooks I, et al. Structural insights into adiponectin receptors suggest ceramidase activity. Nature. 2017, 544(7648): 120-123.
- Tanabe H, et al. Human adiponectin receptor AdipoR1 assumes closed and open structures. Communications Biology. 2020, 3(1): 446.
- Healey R D, et al. An automated platform for structural analysis of membrane proteins through serial crystallography. Cell Reports Methods. 2021, 1(6): 100102.