Time-resolved cryo-electron microscopy (cryo-EM) has transformed structural biology by allowing scientists to observe dynamic molecular processes in unprecedented detail. This cutting-edge technique captures transient, short-lived molecular intermediates, unveiling how biological macromolecules function over time. It represents a major leap forward from traditional cryo-EM, providing insights into the molecular machinery of life.
What is Time Resolved Cryo-EM?
Time-Resolved Cryo-EM is an advanced structural biology technique that combines high temporal and spatial resolution to capture the transient, intermediate states of biological macromolecules during dynamic processes. Unlike traditional cryo-EM, which provides static snapshots of molecules in equilibrium, time-resolved cryo-EM enables the observation of molecular conformational changes in real time as biochemical reactions unfold.
The core principle of time-resolved cryo-EM involves the rapid mixing of reactants, which triggers a biochemical reaction. At precise time intervals, the reaction is halted by plunging the sample into a cryogen, effectively "freezing" the molecule at specific stages of the process. This rapid freezing process, combined with cryo-EM imaging, allows for the reconstruction of high-resolution structures of the molecule at different time points, revealing the dynamic transitions that occur during the reaction.
This technique is particularly valuable for studying molecular machines, such as proteins and nucleic acids, that undergo rapid conformational changes as part of their functional mechanisms. By capturing these fleeting intermediate states, time-resolved cryo-EM provides a deeper understanding of the molecular dynamics that govern critical biological functions, from enzyme catalysis to receptor activation.
Development History of Time-Resolved Cryo-EM
Early Limitations of Cryo-EM (Pre-2000s)
Before the development of time-resolved techniques, traditional cryo-EM faced several challenges. One major issue was the resolution barrier, where film-based imaging resulted in significant signal loss, preventing resolutions better than 3 Å. Additionally, sample preparation was a bottleneck, as early methods lacked the capability to freeze reactions occurring on millisecond timescales. Furthermore, the inability of early algorithms to handle molecular heterogeneity made it difficult to study the intermediate states of dynamic biological processes.
Resolution Revolution in the 2010s
The 2010s marked a pivotal period in cryo-EM's development, characterized by several technological breakthroughs. The introduction of direct electron detectors (DED) replaced traditional film, significantly improving the signal-to-noise ratio and enabling a resolution breakthrough to 2.2 Å. This enhancement was critical for capturing finer molecular details. Additionally, new algorithms such as RELION and cryoSPARC improved the accuracy of image alignment and classification, enabling more precise molecular analysis. The automation of data collection strategies also increased the efficiency and scale of cryo-EM experiments, providing a foundation for future time-resolved studies.
Advancements in Time-Resolved Cryo-EM (Post-2019)
The development of microfluidic mixing devices allowed reactions to be mixed in under 10 milliseconds and sprayed onto a cryo-EM grid for rapid freezing. This achievement enabled millisecond-level time resolution, making it possible to capture dynamic molecular transitions in real-time. This breakthrough was supported by voltage-assisted spray technology, which improved sample distribution and ensured uniform ice thickness, resulting in higher-quality imaging.
Deep Learning and Dynamic Classification Algorithms
One of the significant advancements in time-resolved cryo-EM was the development of dynamic classification algorithms such as AlphaCryo4D and CryoDRGN. These deep learning tools are capable of handling heterogeneous data sets with multiple conformational states, enabling the identification of low-abundance intermediates. These algorithms play a crucial role in revealing the detailed dynamics of biochemical reactions and the conformational changes of macromolecules.
How Does Time-Resolved Cryo-EM Work?
The process of time-resolved cryo-EM can be broken down into four key steps: reaction initiation, reaction termination through rapid freezing, imaging, and data processing. Below is a detailed look at each stage.
1. Triggering the Reaction: Rapid Mixing Technologies
The first critical step in time-resolved cryo-EM is initiating the biochemical reaction of interest. This is achieved by rapidly mixing the reaction components, such as an enzyme and its substrate or a receptor and its ligand. The goal is to capture intermediate molecular states that arise from these reactions within a defined timescale.
- Microfluidic Devices: Specialized microfluidic chips or mixing nozzles are used to mix the reactants within milliseconds. The precise control over mixing allows for accurate synchronization of the reaction start time, ensuring that the molecular process begins at a specific moment.
- Mixing Methods: There are two common methods for triggering the reaction:
1) Laminar Flow Mixing: This technique relies on the diffusion of reactants within a narrow microchannel, achieving precise mixing by ensuring that the layers of fluid come into contact in a controlled manner.
2) Spray Mixing: High-pressure jets are used to spray the reactants onto a support grid. This method is especially useful when extremely rapid reaction initiation is required, reducing the mixing time to microseconds, which is crucial for observing fast molecular events.
Recent breakthroughs in mixing technologies have brought the time scale of reaction initiation down to the microsecond range, enabling the study of faster molecular dynamics.
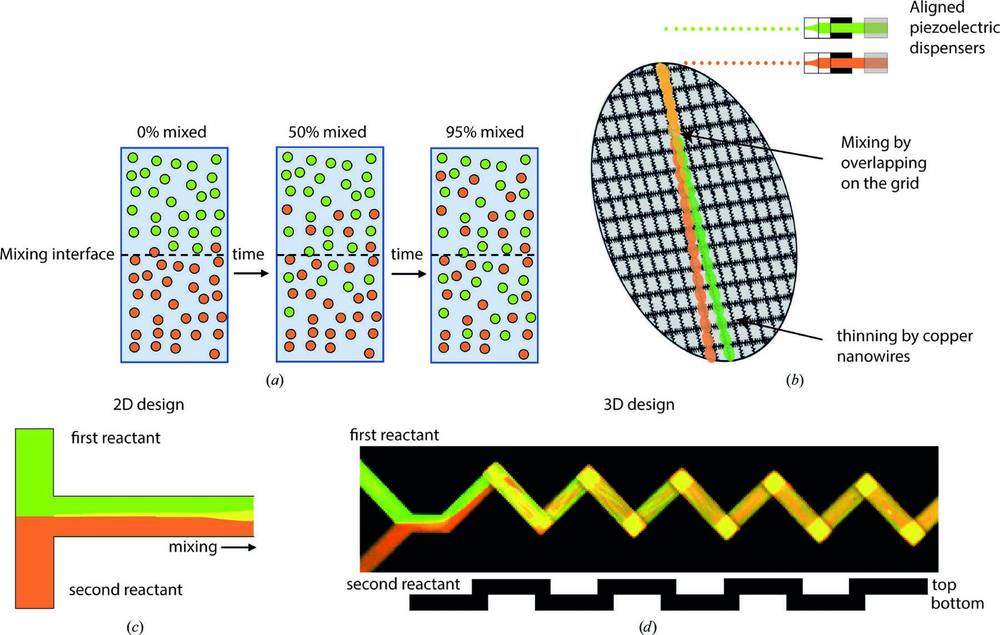 Figure 1. Overview of Time Resolved Cryo-EM Sample-Mixing Techniques. Illustrates various sample-mixing methods: (a) diffusive mixing across a liquid interface, (b) on-grid mixing, (c) parallel lamination mixing, and (d) passive microfluidic mixing. (Mäeots M, et al., 2022)
Figure 1. Overview of Time Resolved Cryo-EM Sample-Mixing Techniques. Illustrates various sample-mixing methods: (a) diffusive mixing across a liquid interface, (b) on-grid mixing, (c) parallel lamination mixing, and (d) passive microfluidic mixing. (Mäeots M, et al., 2022)
2. Terminating the Reaction: Rapid Freezing (Vitrification)
Once the reaction is initiated, the next step is to "freeze" the biological molecules at the desired time point to capture their structure. This is achieved through rapid freezing, also known as vitrification, where the reaction is halted by plunging the sample into a cryogen such as liquid ethane or liquid nitrogen.
- Time Control: The reaction is terminated at precise time intervals (e.g., 10 ms, 100 ms, etc.), with the reaction mixture quickly deposited onto a grid and immediately frozen to preserve the molecular conformations.
- Avoiding Ice Crystal Formation: To preserve the structural integrity of the molecules, it is essential to freeze the sample quickly enough to prevent the formation of ice crystals, which can damage the sample and distort its structure. The freezing process must occur faster than the time it takes for water molecules to rearrange and form ice crystals, typically within milliseconds.
- Sample Thickness: The sample's thickness is typically reduced to around 100 nm to ensure rapid freezing and minimize artifacts. This requires uniformity in the deposition of the sample, as variations in thickness can lead to inconsistent freezing and poor imaging results.
3. Capturing Dynamic Intermediates
The frozen samples now contain molecules in different states corresponding to various stages of the reaction, such as binding, conformational changes, or dissociation. One of the key features of time-resolved cryo-EM is its ability to capture these transient intermediates, which often occur at very low occupancy and cannot be easily observed using traditional methods.
- Multiple Intermediate States: In a single experiment, multiple intermediate states of the molecule can be captured. These states are often highly heterogeneous, with different molecular conformations coexisting within the same sample.
- Time Point Sequences: By conducting the experiment multiple times and freezing the samples at different time points (e.g., 0 ms, 50 ms, 200 ms), researchers can construct a time axis that represents the molecular process in action, allowing for a comprehensive view of the reaction's dynamics.
4. Cryo-EM Imaging and Data Processing
After the samples are frozen, they are imaged using cryo-EM, a technique that uses a transmission electron microscope to capture high-resolution images of the frozen samples. Cryo-EM imaging is performed at low electron doses to prevent radiation damage to the sample.
- Data Collection: Thousands to hundreds of thousands of individual particle images are collected for each time point, creating a large data set of molecular snapshots. These images are then analyzed to reconstruct the three-dimensional structures of the molecules at different stages of the reaction.
- Classification and Reconstruction: Advanced computational techniques, such as single-particle analysis and 3D classification algorithms (e.g., RELION, CryoSPARC), are used to categorize the molecules based on their conformations. This classification process separates different structural states and allows for the determination of the relative abundance of each state at each time point.
- Dynamic Analysis: Once the different states are identified, the next step is to analyze the changes in the molecular conformations over time. The dynamics of the reaction can be quantified by comparing the number of particles in each conformation at different time points, which provides valuable kinetic parameters, such as reaction rates and transition states.
- High-Resolution Structural Insights: The use of time-resolved cryo-EM enables high-resolution reconstructions, often achieving sub-nanometer resolution (3-4 Å). This level of detail allows researchers to observe critical structural changes, such as the movement of key amino acids or the rearrangement of molecular domains during the reaction.
Time-Resolved Cryo-EM vs. Traditional Cryo-EM and Other Techniques
Key Differences Between Time-Resolved Cryo-EM and Traditional Cryo-EM
Time-resolved cryo-EM offers several advantages over traditional cryo-EM by allowing the study of molecular dynamics, capturing the transient states of biological molecules during reactions. Here’s a comparison of the two techniques:
| Feature | Traditional Cryo-EM | Time-Resolved Cryo-EM |
|---|---|---|
| Research Focus | Static or equilibrium structures | Non-equilibrium, transient intermediates |
| Time Dimension | Single-time-point, static imaging | Multi-time-point, dynamic tracking (millisecond resolution) |
| Sample Preparation | Conventional freezing techniques | Microfluidic mixing + rapid freezing |
| Data Analysis | Single classification | Time-sequence classification and particle counting |
| Primary Goal | Structural analysis | Reconstructing dynamics and reaction pathways |
Key Difference: The fundamental distinction is that time-resolved cryo-EM captures dynamic processes by "freezing" them, transforming structural biology from a "snapshot" into a "movie." This is particularly useful for studying fast events, such as molecular switches and allosteric effects, that occur during biochemical reactions.
Comparison with Other Time-Resolved Techniques
| Technique | Advantages | Limitations |
|---|---|---|
| Time-resolved Serial Crystallography | - Time resolution can reach femtosecond levels (e.g., XFEL technology). - Atomic-level resolution (<2 Å). |
- Requires high-quality crystals, making it difficult to study flexible or large complexes. - Radiation damage can affect the accuracy of dynamic observations. |
| Nuclear Magnetic Resonance (NMR) | - Real-time monitoring of solution-state dynamics (milliseconds to seconds). | - Limited to small proteins (usually <50 kDa). - Lower resolution compared to cryo-EM, especially for larger structures. |
| Fluorescence Spectroscopy/FRET | - Highly sensitive, suitable for live-cell environments. | - Provides only local conformational information, lacking global structural details. |
Unique Advantages of Time-Resolved Cryo-EM
Time-resolved cryo-EM combines the benefits of high spatial resolution with the ability to capture dynamic events, offering several advantages over other techniques:
- No Need for Crystallization: Unlike X-ray crystallography, time-resolved cryo-EM does not require crystallization, making it ideal for studying large, flexible complexes such as virus particles and ribosomes.
- Atomic-Level Resolution with Millisecond Time Resolution: Time-resolved cryo-EM bridges the gap between X-ray crystallography and spectroscopic techniques by providing near-atomic resolution while capturing events in real-time over milliseconds. This capability allows researchers to study fast biochemical reactions that are beyond the reach of other methods.
In summary, time-resolved cryo-EM stands out for its ability to observe molecular structures at high resolution while simultaneously tracking dynamic changes over time, providing unparalleled insights into the kinetics and mechanisms of molecular processes.
Select Service
Related Reading
Case Studies: Time-Resolved Cryo-EM in Action
Time-Resolved Cryo-EM in GPCR Activation
Researchers used time-resolved cryo-EM to capture the activation mechanism of G protein-coupled receptors (GPCRs) by visualizing pre-steady-state intermediates of the β2-adrenergic receptor (β2AR)–Gs protein complex at sequential time points after GTP binding. Variability analysis revealed a stepwise conformational trajectory, where structural changes originated from the nucleotide-binding pocket, propagated through the GTPase domain, and altered the Gα Switch regions and α5 helix, ultimately weakening the GPCR-G protein interface. The study reconstructed 20 transition structures, providing high-resolution insights into the activation process. Molecular dynamics simulations further supported that GTP stabilization in later stages correlated with irreversible α5 helix destabilization, leading to G protein dissociation. This research highlights the power of time-resolved cryo-EM in capturing transient molecular events and elucidating complex GPCR signaling mechanisms.
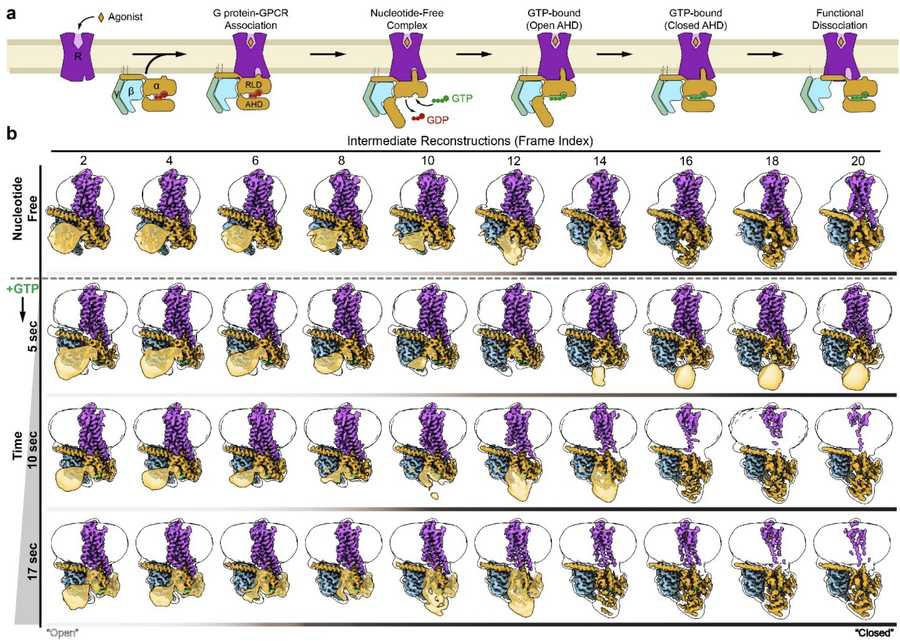 Figure 2. Conformational Dynamics of G Protein Activation. Time-resolved cryo-EM captures the conformational changes during β2AR-Gs activation. (a) GPCR-G protein interaction upon agonist binding. (b) Cryo-EM analysis of β2AR-Gs with AHD closure dynamics over time, showing increased AHD closure as GTP is added. (Papasergi-Scott M M, et al., 2024)
Figure 2. Conformational Dynamics of G Protein Activation. Time-resolved cryo-EM captures the conformational changes during β2AR-Gs activation. (a) GPCR-G protein interaction upon agonist binding. (b) Cryo-EM analysis of β2AR-Gs with AHD closure dynamics over time, showing increased AHD closure as GTP is added. (Papasergi-Scott M M, et al., 2024)
Time-Resolved Cryo-EM in Proteasome Regulation
Time-resolved cryo-EM has been instrumental in uncovering the dynamic regulation of proteasomal degradation, particularly the role of ubiquitin-specific protease 14 (USP14) in modulating proteasome activity. By capturing 13 distinct conformational states of the 26S proteasome-USP14 complex during substrate degradation, researchers identified two parallel pathways governing proteasome state transitions. The technique allowed for the visualization of transient intermediates, revealing how USP14 allosterically reprograms the ATPase motor, facilitates core particle gate opening, and regulates ATP hydrolysis during substrate unfolding. Importantly, time-resolved cryo-EM exposed how USP14-ATPase interactions introduce regulatory checkpoints, controlling ubiquitin recognition, substrate translocation, and ubiquitin recycling. These findings provide critical mechanistic insights into proteasome function and lay the groundwork for the development of USP14-targeted therapeutic strategies.
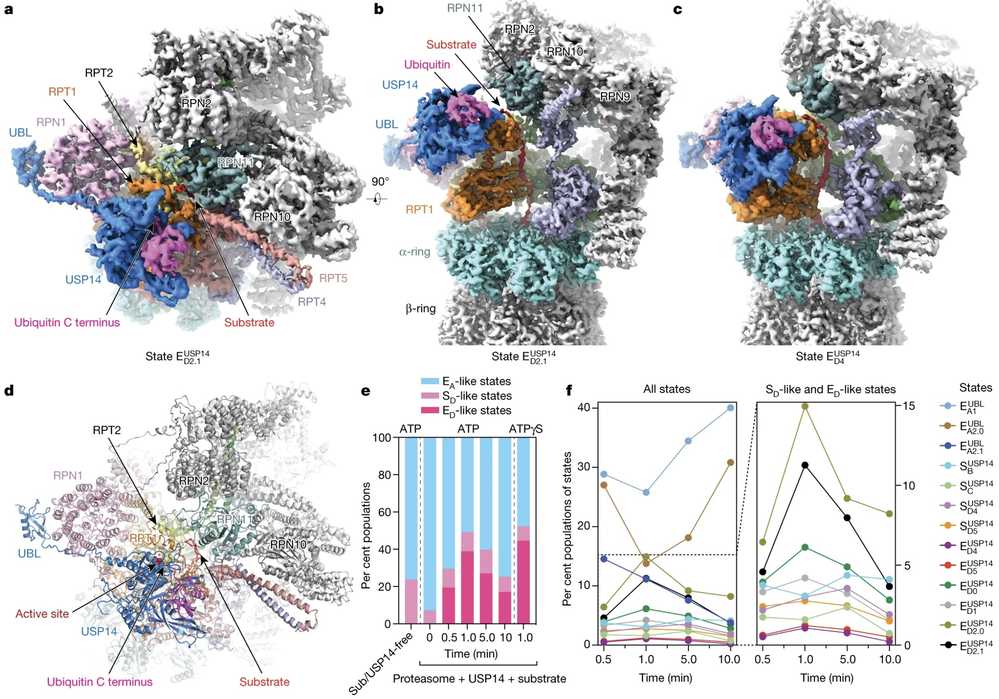 Figure 3. Time-Resolved Cryo-EM Unveils Conformational Changes in USP14-Proteasome Complexes During Substrate Degradation. (a) Top view and (b) side view of the substrate-engaged USP14–proteasome complex. (c) Side view of USP14 binding to the AAA domain of RPT1. (d) Atomic model of the substrate-engaged state. (e, f) Kinetic changes of the different conformational states across time points after substrate mixing. (Zhang S, et al., 2022)
Figure 3. Time-Resolved Cryo-EM Unveils Conformational Changes in USP14-Proteasome Complexes During Substrate Degradation. (a) Top view and (b) side view of the substrate-engaged USP14–proteasome complex. (c) Side view of USP14 binding to the AAA domain of RPT1. (d) Atomic model of the substrate-engaged state. (e, f) Kinetic changes of the different conformational states across time points after substrate mixing. (Zhang S, et al., 2022)
Microsecond Time-Resolved Cryo-EM in Viral Capsid Dynamics
Microsecond time-resolved cryo-EM has enabled researchers to capture ultrafast protein dynamics, providing unprecedented insights into molecular function. Using this technique, scientists studied the cowpea chlorotic mottle virus (CCMV) capsid, observing how a pH change triggers rapid contraction within microseconds. While the transition occurs as a whole, individual capsid proteins move at different speeds, revealing a complex reaction pathway. Such detailed visualization would be difficult with other methods, underscoring the unique power of microsecond time-resolved cryo-EM. This breakthrough expands the potential of cryo-EM for studying previously unobservable rapid biomolecular processes.
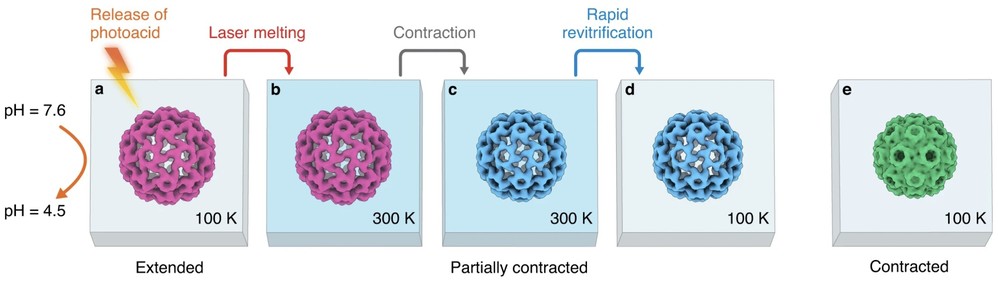 Figure 4. Microsecond Time-Resolved Cryo-EM Reveals CCMV Contraction Dynamics. (a) CCMV expanded form is frozen at pH 7.6 with a photoacid. (b) UV irradiation lowers the pH to 4.5, trapping the virus in its pre-contracted state. (c) Laser irradiation melts the sample, initiating capsid contraction. (d) Rapid cooling and revitrification traps the virus in intermediate states. (e) Fully contracted virus structure obtained at pH 5.0. (Harder O F, et al., 2023)
Figure 4. Microsecond Time-Resolved Cryo-EM Reveals CCMV Contraction Dynamics. (a) CCMV expanded form is frozen at pH 7.6 with a photoacid. (b) UV irradiation lowers the pH to 4.5, trapping the virus in its pre-contracted state. (c) Laser irradiation melts the sample, initiating capsid contraction. (d) Rapid cooling and revitrification traps the virus in intermediate states. (e) Fully contracted virus structure obtained at pH 5.0. (Harder O F, et al., 2023)
Future Directions of Time-Resolved Cryo-EM
High Data Volume and Computational Challenges
Each time point in time-resolved cryo-EM requires capturing tens to hundreds of thousands of particles, making data collection and processing time-intensive and costly. Advanced computational strategies are needed to handle and analyze these massive datasets more efficiently.
Improving Mixing Uniformity
Microfluidic devices used in time-resolved cryo-EM rely on laminar flow, which can cause uneven reaction time distributions. Enhancing microfluidic mixing precision will improve the accuracy of molecular state capture, leading to more reliable structural insights.
Ultrafast Reaction Initiation
Future developments aim to reduce the reaction window further using femtosecond laser-triggered reactions or light-sensitive molecular probes. These techniques will enable the study of even faster biochemical processes with greater temporal precision.
Advanced Computational Methods
The integration of manifold embedding techniques and AI-driven analysis will allow researchers to reconstruct continuous conformational landscapes from a single dataset. This approach will reduce the need for excessive sampling while improving the resolution of dynamic molecular changes.
In Situ Time-Resolved Cryo-EM
Combining time-resolved cryo-EM with cellular cryo-preservation techniques will allow researchers to observe molecular dynamics within their native physiological environment, bridging the gap between in vitro and in vivo studies.
In summary, time-Resolved Cryo-EM, with its high-resolution spatiotemporal analysis, is revolutionizing the study of dynamic molecular mechanisms. Its successful applications in areas such as GPCR signaling and viral entry highlight its irreplaceable role in uncovering the "molecular movie" of life processes. As advancements in mixing technologies, algorithms, and hardware continue, this technique promises to play an even greater role in drug design, including the development of inhibitors targeting intermediate states, and in synthetic biology, such as the design of dynamic molecular machines. At Creative Biostructure, we offer comprehensive cryo-EM solutions, including time-resolved analysis, to accelerate your research. Contact us to explore how our solutions can enhance your research projects.
References
- Mäeots M E, Enchev R I. Structural dynamics: review of time-resolved cryo-EM. Biological Crystallography. 2022, 78(8): 927-935.
- Zhang S, Zou S, Yin D, et al. USP14-regulated allostery of the human proteasome by time-resolved cryo-EM. Nature. 2022, 605(7910): 567-574.
- Klebl D P, Aspinall L, Muench S P. Time resolved applications for Cryo-EM; approaches, challenges and future directions. Current Opinion in Structural Biology. 2023, 83: 102696.
- Harder O F, Barrass S V, Drabbels M, et al. Fast viral dynamics revealed by microsecond time-resolved cryo-EM. Nature Communications. 2023, 14(1): 5649.
- Papasergi-Scott M M, Pérez-Hernández G, Batebi H, et al. Time-resolved cryo-EM of G-protein activation by a GPCR. Nature. 2024, 629(8014): 1182-1191.

-1.jpg)