Structural Research of Nucleotidyltransferases
Nucleotide transferases are enzymes that catalyze the transfer of nucleotide monophosphate groups between nucleotide molecules. They play a crucial role in various biochemical processes, including synthesizing, repairing, and modifying DNA and RNA. Nucleotide transferases are also involved in the metabolism of nucleotides, such as the biosynthesis of nucleotides and nucleotides from precursor molecules. X-ray crystallography enables scientists to determine the three-dimensional structure of nucleotide transferase. This can help reveal how these enzymes work at the molecular level and understand their mechanisms of action.
Translator assembly and maintenance protein 41 (Tam41, also known as MMP37) is a peripheral membrane protein facing the matrix side in the inner mitochondrial membrane. Tam41 is believed to be involved in the biosynthesis of CDP-DAG and cardiolipin in mitochondria.
To deepen the detailed understanding of the biosynthesis mechanism of CDP-DAG in mitochondria, researchers characterized the crystal structure of Tam41 from Schizosacharomyces pombe in the N-terminal region of apo and CTP binding states.
Analysis of the crystal structure indicates that Tam41 uses a sequential catalytic mechanism, consisting of a nucleotide transferase (NTase) domain and a winged helical domain. The CTP is combined into an "L" shaped pocket between two structural domains, and rearranging a circular area is necessary for opening the pocket. Phosphatidic acid/CDP-DAG uses a lipid entry/exit pathway linked to the pocket. The C-terminal region of Tam41 contains an amphoteric helix crucial for membrane binding and phospholipid binding.
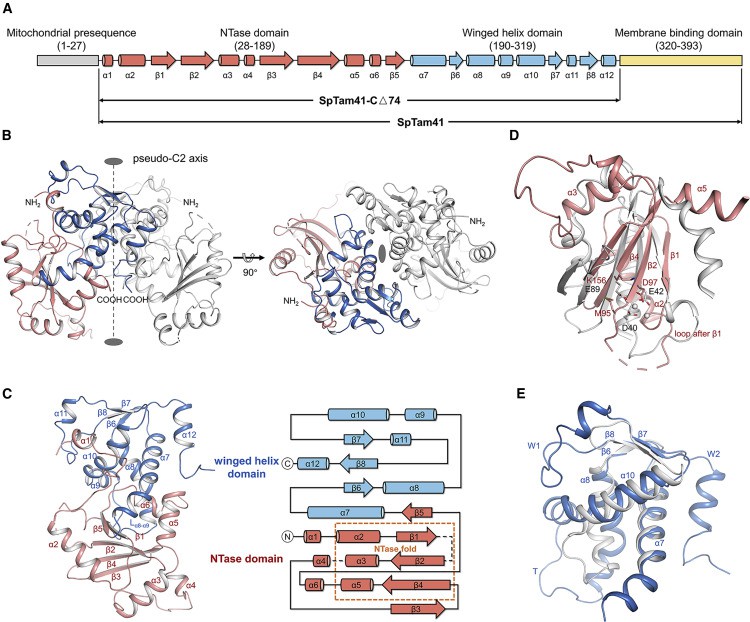 Figure 1. SpTam41 Contains an NTase Domain and a Winged Helix Domain. (JIAO, et al., 2019)
Figure 1. SpTam41 Contains an NTase Domain and a Winged Helix Domain. (JIAO, et al., 2019)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Tam41 mitochondrial CDP-DAG synthase Δ74 | Schizosaccharomyces pombe 972h- | X-ray diffraction | 2.26 Å | 6IG4 |
| Tam41 mitochondrial CDP-DAG synthase Δ74 F240A mutant | Schizosaccharomyces pombe 972h- | X-ray diffraction | 2.88 Å | 6IG2 |
Table 1. Structural research of nucleotidyltransferases.
X-ray crystallography is an effective method to determine the molecular structure of biological macromolecules through diffraction patterns, which reveal the three-dimensional structure and function of biological macromolecules. At Creative Biostructure, we primarily provide reliable X-ray crystallography services and technical support for life science research. We utilize high-intensity X-ray and precise detectors to obtain reliable, accurate, high-resolution structural data. Our expert team will work with you to develop the most suitable solution for your research requirements. We are determined to provide excellent services to make significant progress in biomolecular research.
Reference
- JIAO H, et al. Structures of the mitochondrial CDP-dag synthase tam41 suggest a potential lipid substrate pathway from membrane to the active site. Structure, 2019, 27(8).
