Structure and Function of Membrane Proteins
Membrane proteins are essential for cellular functions, acting as gateways for the exchange of substances and information across cellular membranes. Embedded in the lipid bilayer, these proteins exhibit intricate structural organization that underlies their diverse roles. They are involved in various critical processes, including transport, signal transduction, and cell adhesion. Understanding their structure is crucial for deciphering their function and potential as drug targets.
Tertiary Structure of Membrane Proteins
The tertiary structure of a membrane protein refers to the three-dimensional arrangement of its polypeptide chain. This structure is stabilized by various interactions such as hydrogen bonds, disulfide bridges, ionic bonds, and hydrophobic interactions. The tertiary structure is crucial for the protein's functionality as it defines the spatial architecture required for the protein to interact with other cellular components and perform its specific role.
Folding Patterns
Alpha-Helices: Many membrane proteins adopt an alpha-helical conformation which allows them to span the hydrophobic core of the lipid bilayer. These helices can bundle together to form channels or transporters, allowing selective passage of ions or molecules. Alpha-helical proteins are predominantly found in the plasma membrane.
Beta-Barrels: Beta-barrel proteins are primarily found in the outer membranes of mitochondria, chloroplasts, and Gram-negative bacteria. They consist of beta-strands that create a cylindrical structure, facilitating molecule passage across the membrane. The beta-barrel configuration provides a structural scaffold essential for their function.
Stabilizing Interactions
Hydrogen Bonds: Within the protein backbone, hydrogen bonds play a critical role in stabilizing the tertiary structure. These bonds occur between the carbonyl oxygen and amide hydrogen atoms of the peptide backbone.
Hydrophobic Interactions: The hydrophobic effect significantly contributes to stabilizing the folded structure within the lipid bilayer. Hydrophobic side chains of amino acids tend to cluster together in the interior of the protein, minimizing their exposure to the aqueous environment.
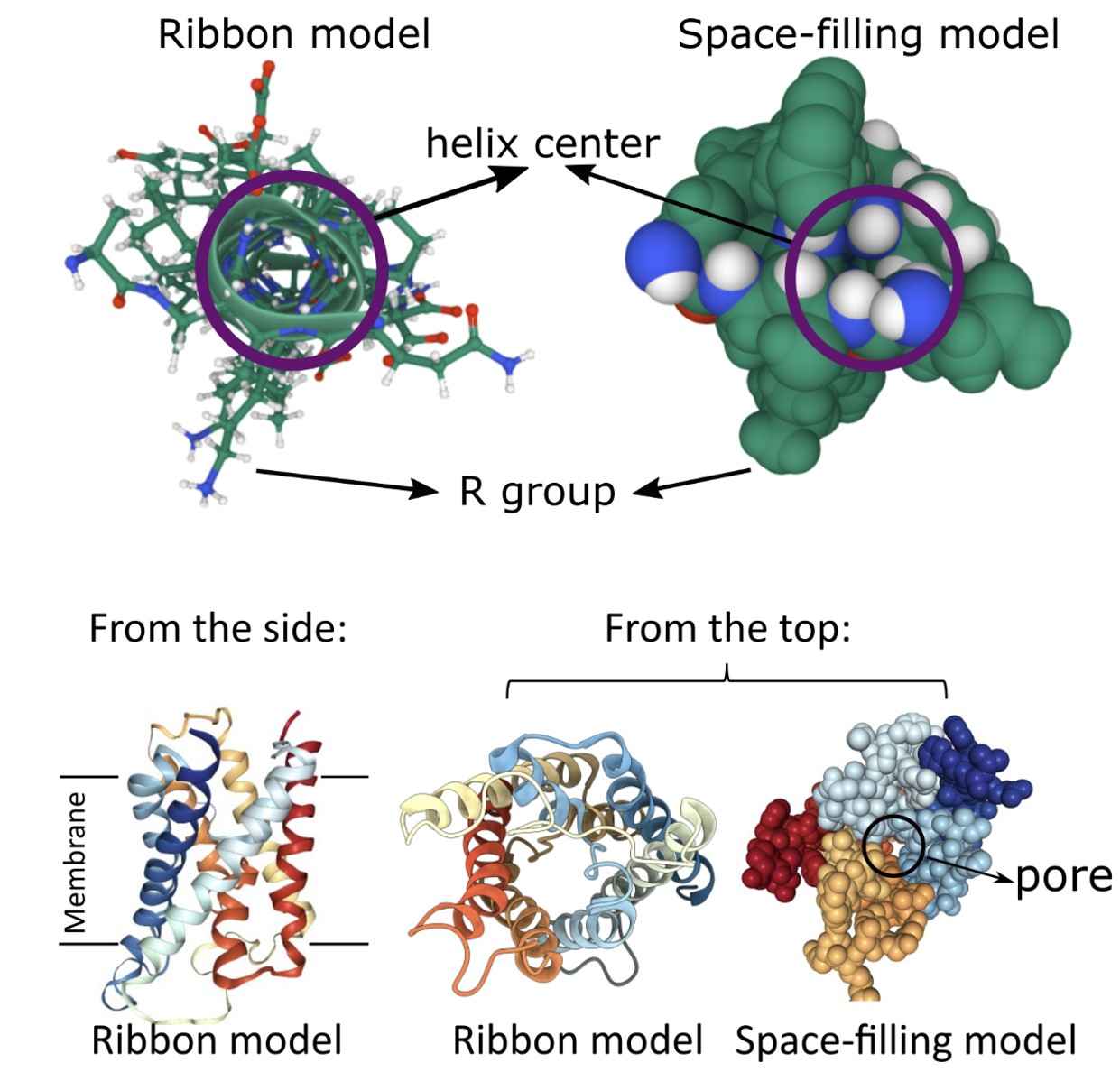 Figure 1. A single alpha helix and the protein structure of aquaporin. (Lauren Dalton)
Figure 1. A single alpha helix and the protein structure of aquaporin. (Lauren Dalton)
Quaternary Structure of Membrane Proteins
The quaternary structure refers to the assembly of multiple polypeptide chains (subunits) into a larger, functional complex. This structure is vital for the functionality and stability of membrane proteins, often enhancing their performance and allowing them to undertake more complex roles. Subunits in a quaternary structure can interact through non-covalent forces or, less frequently, covalent modifications.
Types of Quaternary Structures
Homo-oligomers: Composed of identical subunits, homo-oligomers are symmetric assemblies often observed in ion channels, where multiple identical subunits create a pore for ion passage.
Hetero-oligomers: These consist of different but functionally complementary subunits. Hetero-oligomers can include GPCRs (G-protein coupled receptors), which operate as multi-subunit complexes, enabling signal transduction from extracellular ligands to intracellular pathways.
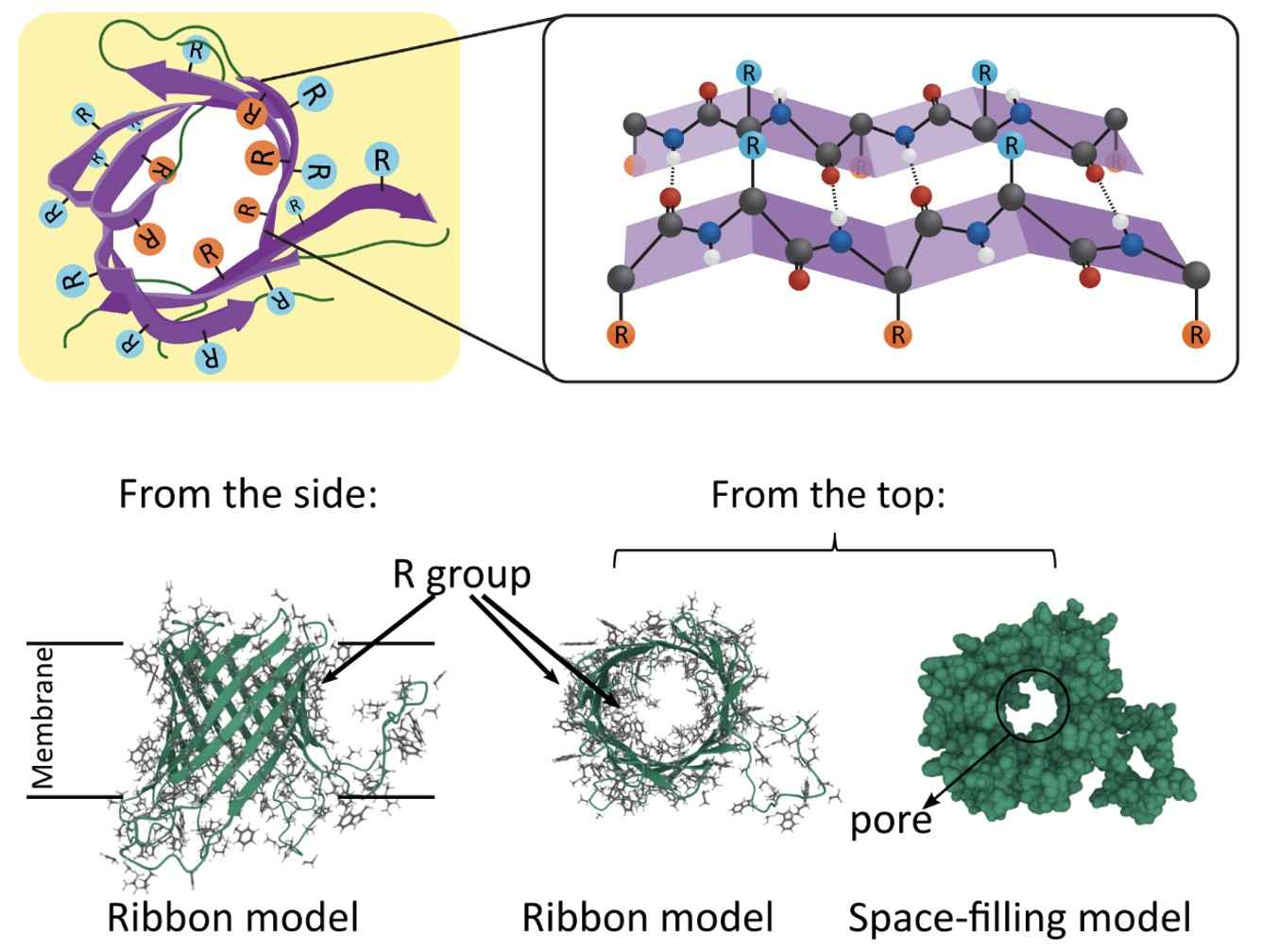 Figure 2. Beta sheet structure within Beta barrels and the structure of bacterial outer membrane protein G. (Lauren Dalton)
Figure 2. Beta sheet structure within Beta barrels and the structure of bacterial outer membrane protein G. (Lauren Dalton)
Functional Significance of Quaternary Structures
The quaternary structure is essential for the cooperative function of membrane proteins. For instance, ion channels have multiple subunits forming a pore that allows ion flow. The multi-subunit arrangement facilitates regulation and fine-tuning of the channel's activity, as subunits can modulate the response to different stimuli. GPCRs operate in a quaternary structure, transmitting signals through coordinated interactions between subunits, which initiate various cellular responses.
Transmembrane Regions and Their Roles
Transmembrane (TM) domains are critical for the proper insertion and function of membrane proteins within the lipid bilayer. These regions exhibit specific characteristics that influence the protein's stability and functionality.
Characteristics of Transmembrane Regions
Alpha-helical transmembrane regions are characterized by helical segments that span the bilayer with hydrophobic side chains outwardly facing the lipid environment. These segments can vary in length and hydrophobicity, influencing the orientation and insertion of the protein within the membrane. Beta-barrel transmembrane regions consist of beta-strands that form a cylindrical structure, allowing passage through the membrane while maintaining stability via extensive hydrogen bonding.
Length and Composition: TM domains vary in length and amino acid composition, affecting their hydrophobicity and function. They typically consist of hydrophobic amino acids that interact favorably with the lipid environment.
Glycine and Proline: These amino acids are often found in TM regions due to their flexibility and ability to introduce kinks in the helix. Glycine provides conformational flexibility, while proline can introduce bends or disruptions in alpha-helical structures, influencing the protein's overall shape and orientation within the membrane.
Functional Roles of Transmembrane Regions
- Barrier to Solutes: TM domains serve as a barrier to the passage of polar molecules across the membrane, maintaining the integrity and distinct environment of the cellular compartments.
- Conduction Pathways: In channels and transporters, TM domains form the pathways for ion or molecule transport. For example, ion channels rely on alpha-helical TM segments to create a selective and gated pathway for ions to flow through, determining the channel's selectivity and response to stimuli.
- Signal Transduction: TM regions facilitate signal transduction by transmitting external signals into the cell. This is evident in receptor proteins that undergo conformational changes upon ligand binding, leading to intracellular signaling cascades.
- Anchoring and Enzymatic Activity: TM domains anchor proteins to the membrane, ensuring their proper localization. Some enzymes embedded in the membrane use TM regions to position their active sites optimally for substrate interaction.
High-Resolution Microscopy in Membrane Protein Research
In the study of membrane proteins, high-resolution microscopic techniques play a crucial role. These methods allow researchers to visualize membrane proteins at near-atomic resolution, providing deep insights into their structure and function.
Cryo-electron microscopy (Cryo-EM) has become the mainstream method for determining high-resolution structures of membrane proteins, especially for those that are difficult to crystallize. By rapidly freezing samples to maintain their structures in a near-physiological state, Cryo-EM can provide near-atomic resolution.
| Techniques | Description |
| Single Particle Analysis (SPA) | A branch of Cryo-EM, SPA is suitable for samples that cannot form crystals. By computationally averaging images of many individual protein particles, a three-dimensional structure can be reconstructed. |
| Time-Resolved Cryo-EM | This method involves flash-freezing biological samples at specific time intervals during dynamic conformational changes and visualizing them using cryo-electron microscopy. |
| Electron Crystallography | This technique involves creating two-dimensional crystals of membrane proteins and using electron microscopy to collect their diffraction patterns for three-dimensional structural analysis. Although this technique requires high-quality sample preparation, it has been successfully used to resolve the structures of some membrane proteins. |
| Electron Tomography | By acquiring two-dimensional images of a sample from multiple angles and reconstructing them into a three-dimensional model, electron tomography can provide detailed structural information of membrane proteins in their cellular environment. |
| Negative Stain Electron Microscopy | A quick screening technique for membrane protein samples, this method enhances contrast by applying a layer of heavy metal salts around the sample. Although the resolution is relatively low, it helps in the preliminary assessment of sample quality. |
| Special Sample Preparation Techniques | Improving sample quality and reducing background noise are crucial for high-resolution imaging of membrane proteins. Techniques such as using Amphipol or Nanodiscs have been developed to enhance image clarity. |
Membrane Protein Structural Research
We present a comprehensive list of various resolved structures for membrane proteins. This curated compilation allows you to explore advancements in the structural studies of different membrane protein families or specific membrane proteins of interest. By delving into these detailed structural data, researchers can gain valuable insights into the intricate mechanisms underlying membrane protein function, facilitating a deeper understanding and furthering the potential for targeted drug design and therapeutics development.
Creative Biostructure offers membrane-associated protein products, custom membrane protein production services and advanced techniques for membrane protein structural characterization to support your research needs. Our commitment is to accelerate your research and drug development projects by providing precise and reliable membrane protein solutions. If you are interested in our services and products, please feel free to contact us for more information.