Structural Research of Fatty Acid Desaturases
Fatty acid desaturase (FAD) is an enzyme that introduces double bonds into the fatty acyl chains, which is a crucial process in the biosynthesis of essential polyunsaturated fatty acids. FAD plays an important role in regulating membrane fluidity, energy metabolism, and the synthesis of lipid-derived signaling molecules. Dysregulation of FADs has been associated with various diseases such as obesity, diabetes, and cancer. Therefore, understanding the structure of FAD is essential for developing therapeutic approaches for these diseases.
Stearoyl-CoA desaturase 1 (SCD1) is a well-studied FAD that plays a key role in lipid metabolism as well as the development of metabolic diseases and cancer. Recent studies have provided important insights into the structure and function of SCD1. For instance, crystal structures of mouse SCD1 have been reported, which revealed the presence of a diiron center in the enzyme catalytic site. The normal function of SCD1 requires this diiron center and an electron transfer chain consisting of NADH or NADPH, cytochrome b5 reductase, and cytochrome b5.
In addition, crystal structures of human SCD1 and its substrate, stearoyl-CoA, have been determined, which help to elucidate the determinants of substrate binding and the catalytic mechanism of desaturation of the stearoyl portion. This structure also provides a mechanism for the localization of SCD1 in the endoplasmic reticulum, which folds around a tight hydrophobic core composed of four long α-helices that act as anchors spanning the endoplasmic reticulum membrane.
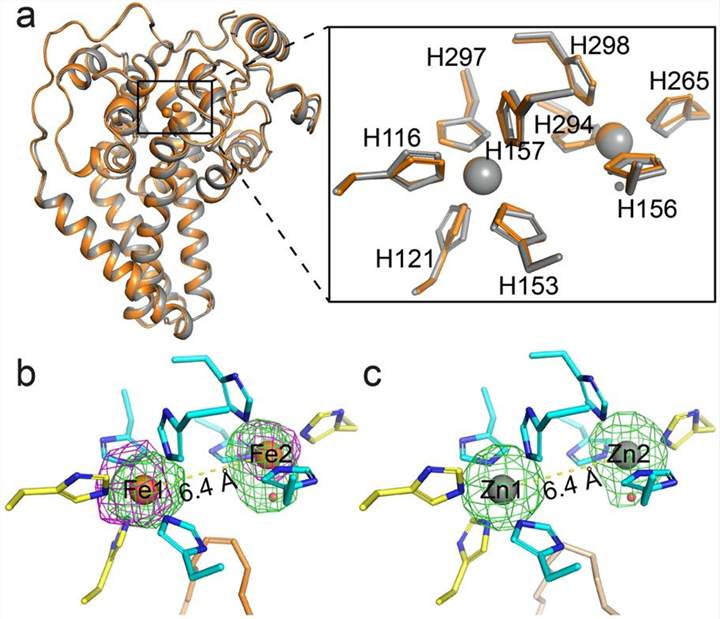 Figure 1. Crystal structure of iron-containing mouse SCD1. (Shen J, et al., 2020)
Figure 1. Crystal structure of iron-containing mouse SCD1. (Shen J, et al., 2020)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Stearoyl-coenzyme A desaturase (SCD1) in complex with substrate (expressed in Spodoptera frugiperda) | Homo sapiens | X-ray diffraction | 3.25 Å | 4ZYO |
| Stearoyl-coenzyme A desaturase (SCD1) in complex with substrate (expressed in Trichoplusia ni) | Mus musculus | X-ray diffraction | 2.60 Å | 4YMK |
| Stearoyl-coenzyme A desaturase (SCD1) with diiron center (expressed in HEK293 cells) | Mus musculus | X-ray diffraction | 3.51 Å | 6WF2 |
Table 1. Structural Research of Fatty Acid Desaturases.
At Creative Biostructure, we provide comprehensive structural analysis services to help researchers better understand the complex biological systems involved in fatty acid desaturation. Our team of experts uses state-of-the-art techniques, such as X-ray crystallography, to determine the 3D structures of proteins involved in this process.
X-ray crystallography is a highly effective scientific technique used to determine the precise atomic-level structures of proteins and other biomolecules. We offer X-ray crystallography services that cater to the needs of researchers involved in the entire drug discovery and development process, ranging from target identification and validation to lead optimization and beyond. We understand that every research project is unique, and hence, we provide a wide range of X-ray crystallography services that are customized to meet your specific requirements. Our team of skilled crystallographers can collaborate with you to design experiments and optimize conditions to produce high-quality protein crystals, which are suitable for structure determination. We also provide crystallography data collection and processing services, utilizing cutting-edge equipment and software.
Whether you are studying the basic biology of fatty acid desaturases or developing new drugs to treat metabolic diseases and cancers, we have the expertise and resources to help you achieve your goals. Contact us today to learn more about our structural analysis services and how we can help you advance your research.
References
- Bai Y, et al. X-ray structure of a mammalian stearoyl-CoA desaturase. Nature. 2015, 524(7564): 252-256.
- Wang H, et al. Crystal structure of human stearoyl–coenzyme A desaturase in complex with substrate. Nature Structural & Molecular Biology. 2015, 22(7): 581-585.
- Shen J, et al. Structure and mechanism of a unique diiron center in mammalian stearoyl-CoA desaturase. Journal of Molecular Biology. 2020, 432(18): 5152-5161.