Exosome Isolation by Ultrafiltration (UF)
Exosomes are heterogeneous biological particles released by cells and are an attractive source of potential biomarkers for early detection of diseases such as cancer. Rapid, simple, high-purity and high-recovery isolation methods are the first prerequisite for exosome-related research and the large-scale application of exosomes in medical practice.
Ultrafiltration (UF) is a size-based exosome isolation technology using membranes with molecular weight cut-offs (MWCO) in the range of 10-100kDa.The UF process is simple, does not require additional special equipment, and allows for the isolation of exosomes with well-defined particle sizes by adjusting the pore sizes of the filter membranes. It is reported that UF has the highest recovery for exosome particles smaller than 100nm, and it improves yield and separation efficiency with a shorter processing time compared to Ultracentrifugation (UC).
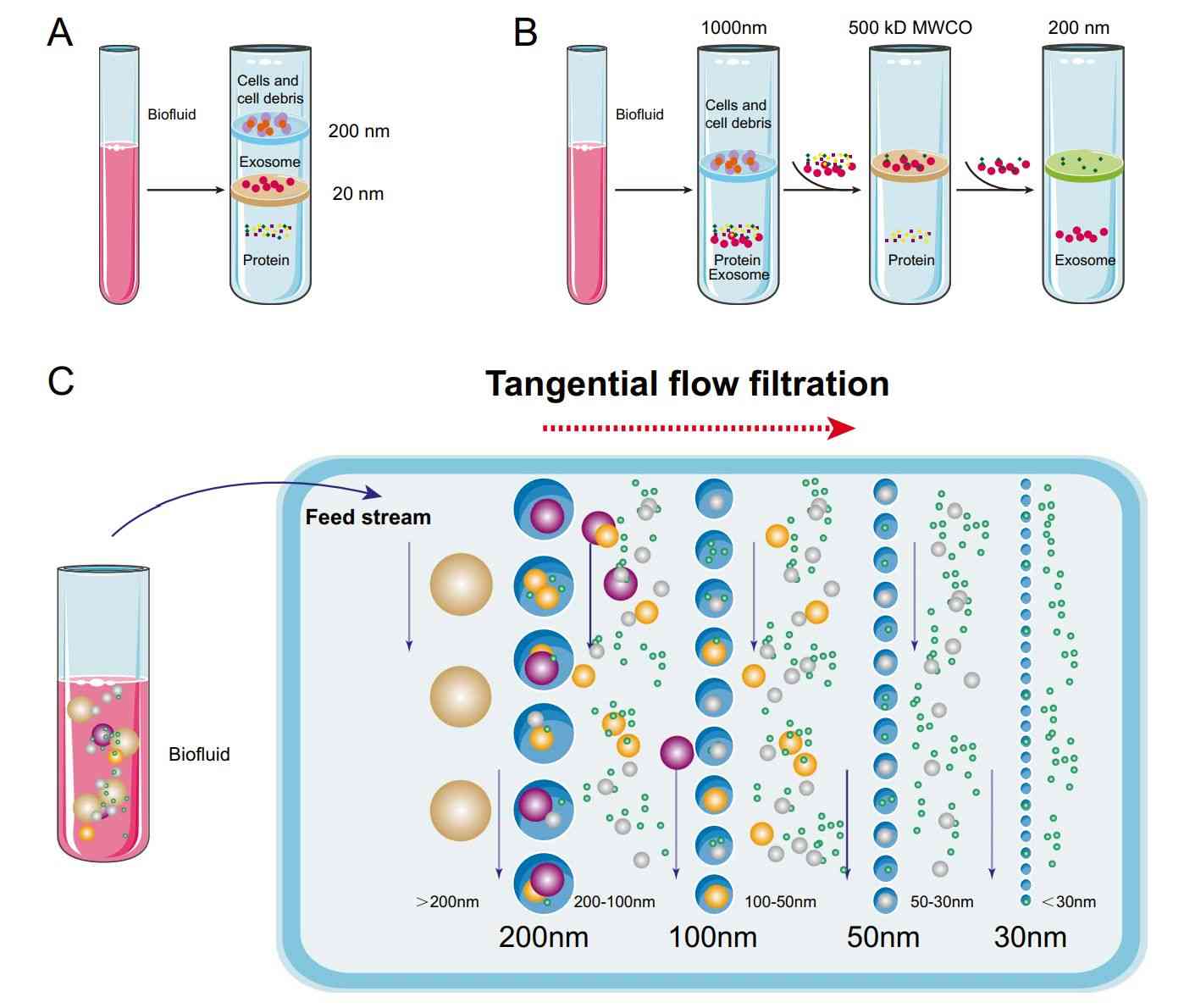 Figure 1. Schematic of ultrafiltration-based exosome isolation. (Liu WZ, et al., 2022)
Figure 1. Schematic of ultrafiltration-based exosome isolation. (Liu WZ, et al., 2022)
How does Ultrafiltration (UF) Work?
1. Sample preparation - Collect samples such as cell culture supernatant and biofluids (e.g. plasma or urine). Centrifuge to remove cellular debris and larger particles.
2. Selection of ultrafiltration membranes - Select an ultrafiltration membrane with specific MWCO based on the size range of exosomes to be separated.
3. Filtration - The ultrafiltration membrane acts as a barrier, retaining exosomes while allowing smaller molecules (e.g., proteins, nucleic acids) and solvents to pass through.
4. Wash - After initial filtration, a wash step may be performed to remove residual contaminants or unbound material.
5. Elution - Add elution buffer to the device and apply pressure or centrifuge to recover exosomes.
Advancement of Ultrafiltration-Based Exosome Isolation
Exosomes may cause the pores of the filter membrane to become clogged as they pass through the membrane, increasing costs. Shear forces can also disrupt the integrity of exosomes. Therefore, researchers then developed tangential flow filtration (TFF), where particles flowing along the membrane are parallel to the membrane surface, rather than flowing toward or perpendicular to the membrane, which can skillfully avoid the clogging problem. Based on this, a recirculating tangential flow filtration (TFF) system was proposed, which has higher isolation purity than single-circulation TFF and also better ensures the integrity of exosomes' structural and biological functions.
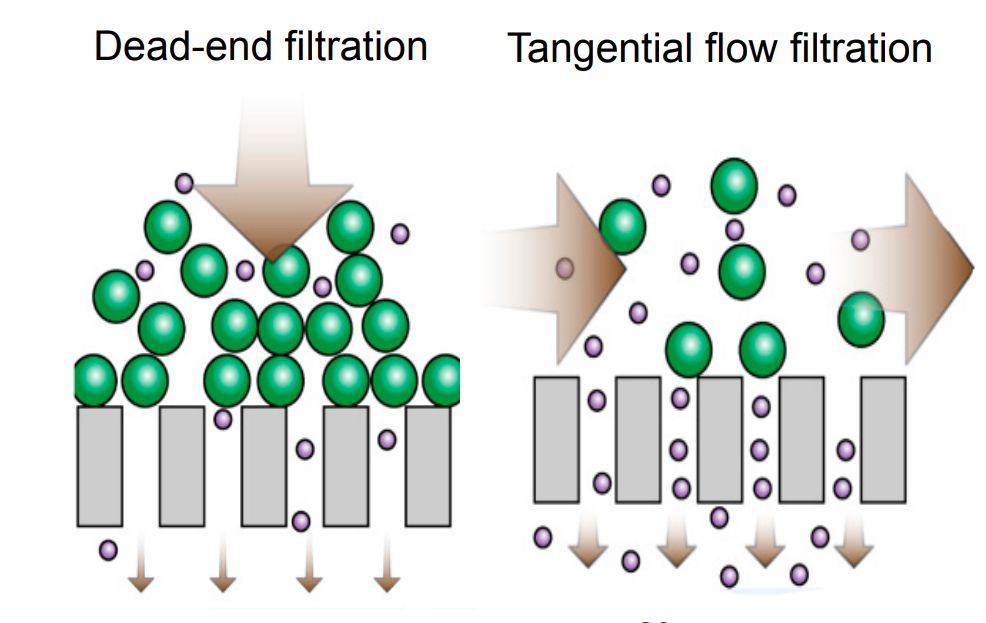 Figure 2. Schematic illustrating the difference between dead-end filtration and TFF. (Busatto S, et al., 2018)
Figure 2. Schematic illustrating the difference between dead-end filtration and TFF. (Busatto S, et al., 2018)
In addition, researchers have proposed a new exosome isolation technology, hydrostatic pressure filtration dialysis, which can concentrate samples and accommodate large sample volumes. The effect of soluble proteins on the purity of exosomes can also be eliminated. In recent years, UF has been applied in combination with other methods, such as UF-LC ultrafiltration combined with size-exclusion Chromatography (SEC), ultrafiltration-based technology for sEVs total isolation chip, and ultrafiltration-TiO2 series methods. The complete and high-purity exosomes obtained can be used for subsequent characterization, in vivo, and in vitro functional analysis.
Why Choose Ultrafiltration (UF) to Isolate Exosomes?
UF technology offers multiple advantages for exosome separation, including speed, simplicity, and the ability to process large sample volumes. It selectively filters particles based on size, which is especially helpful for isolating exosomes that typically range from 30-150nm. UF technology also removes contaminants such as proteins and lipoproteins that hamper the downstream analysis of exosomes. Additionally, UF technology is less harmful to exosomes and maintains their structural and functional integrity.
Applications
- Concentrate dilutions before exosome isolation.
- Easy removal of other small molecules and ions.
- Exosome dialysis and buffer exchange.
- Combined with SEC columns for efficient separation of my exosomes.
In addition to UF-based exosome isolation services, we also offer exosome kits for efficient, easy-to-operate isolation of exosomes. We also have a range of high-purity exosome products to help clients explore the potential applications of exosomes in the treatment and diagnosis of cancer and chronic diseases. Below are examples of our products:
| Cat No. | Product Name | Source |
| Exo-CH16 | HQExo™ Exosome-COLO1 | Exosome derived from human colon carcinoma (COLO1 cell line) |
| Exo-CH10 | HQExo™ Exosome-HCT116 | Exosome derived from human colorectal carcinoma cell line (HCT116 cell line) |
| Exo-SC03 | HQExo™ Exosome-hTERT | Exosome derived from hTERT-immortalized Mesenchymal Stem Cell |
| Exo-SC02-2 | HQExo™ Exosome-Pla-MSC | Exosome derived from human placental derived mesenchymal stem cell |
| Exo-IC01 | HQExo™ Exosome-BC3 | Exosome derived from human B lymphocyte cell line (BC-3 ) |
| Exo-IC05 | HQExo™ Exosome-JAWSII | Exosome derived from mouse bone marrow immature dendritic cell line (JAWSII) |
| Exo-GC05 | HQExo™ Exosome-CHO | Exosome derived from Chinese Hamster Ovary cell (CHO cell line) |
| Exo-GC03 | HQExo™ Exosome-GFP | Exosome derived from HEK293 cell line with GFP loaded |
| Explore All Exosome Products | ||
Creative Biostructure has been following the progress of exosome research for many years. With expertise in exosome isolation, purification, analysis, and application development, we provide strong support to our clients. If you are looking for high-quality exosome products and reliable exosome isolation services, do not hesitate to contact us.
References
- Liu WZ, et al. Current status and outlook of advances in exosome isolation. Anal Bioanal Chem. 2022. 414(24): 7123-7141.
- Busatto S, et al. Tangential Flow Filtration for Highly Efficient Concentration of Extracellular Vesicles from Large Volumes of Fluid. Cells. 2018. 7(12): 273.
- Jia Y, et al. Small extracellular vesicles isolation and separation: Current technologys, pending questions and clinical applications. Theranostics. 2022. 12(15): 6548-6575.