Plant-derived Exosomes in Agriculture
Pathogens and pests wreak havoc on plant health and threaten agriculture and natural ecosystems. Communication between plant cells and interacting pathogens/pests requires functional molecules to enter and leave the extracellular environment. Exosomes are lipid bilayer-encapsulated extracellular vesicles that deliver RNA, proteins, and metabolites from donor cells to recipient cells and participate in cellular processes. Emerging evidence suggests that plant-derived exosomes are important in cross-border molecular exchanges between donor cells and interacting microorganisms to regulate plant immunity and pathogen virulence.
- In production, exosomes can be used to study plant-environment interactions, to understand the mechanisms by which plants adapt to environmental changes, and to provide new ideas and methods for breeding more resilient and productive crops.
- In basic research, studying exosomes will help to deeply understand the interaction mechanism between plants and the external environment, reveal the inner life activity law, and promote the continuous development of plant science.
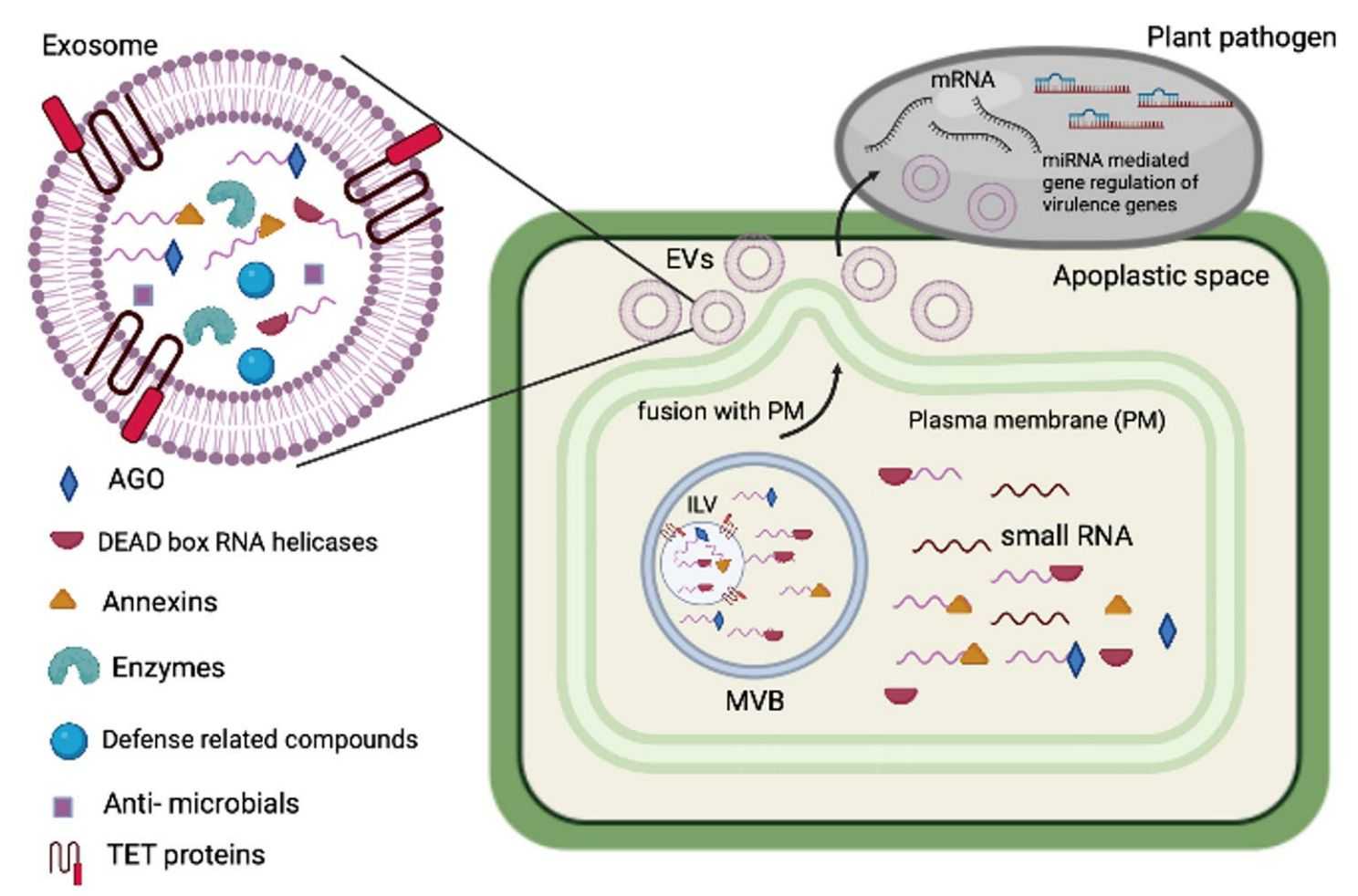 Figure 1. Schematic of plant-derived exosomes against pathogens. (Subha D, et al., 2023)
Figure 1. Schematic of plant-derived exosomes against pathogens. (Subha D, et al., 2023)
Isolation and Characterization of Plant-derived Exosomes
Isolation of exosomes from a large subpopulation of extracellular vesicles (EVs) is challenging due to similar size and morphology. For the isolation of exosomes in plants, the differential ultracentrifugation (dUC) technique is widely used. Due to its simplicity and affordability, this method has become the gold standard for the isolation and purification of plant-derived exosomes. However, exosomes obtained after the dUC procedure are usually contaminated with nucleic acids and proteins. Therefore, for further purification, density gradient ultracentrifugation is required.
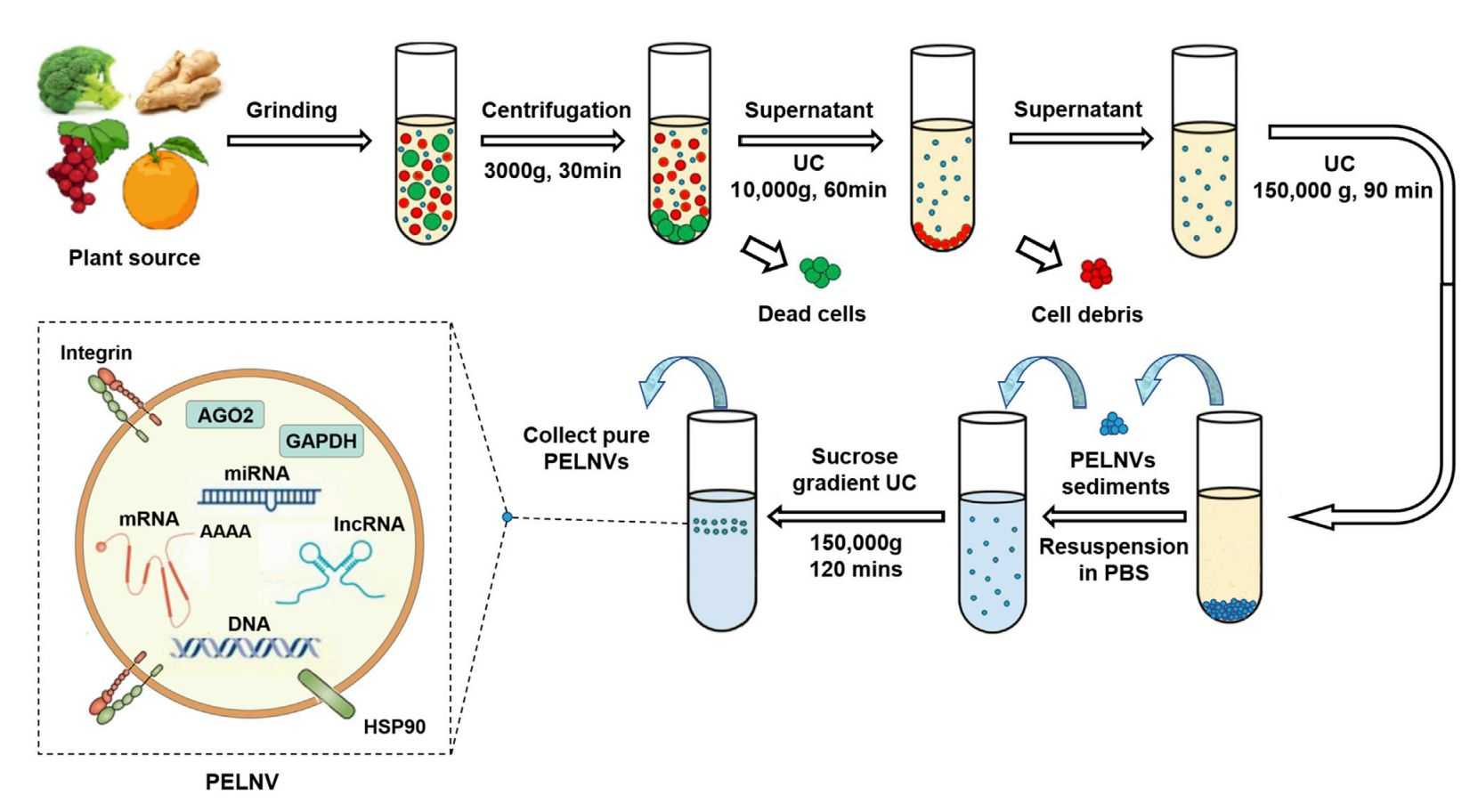 Figure 2. Process for isolation and preparation of plant-derived exosomes. (Dad HA, et al., 2021)
Figure 2. Process for isolation and preparation of plant-derived exosomes. (Dad HA, et al., 2021)
Similar to animal-derived exosomes, the physical properties of plant-derived exosomes can be recognized by particle size distribution, concentration, and morphology. Traditional methods include nanoparticle tracking analysis (NTA), dynamic light scattering (DLS), flow cytometry, and transmission electron microscopy (TEM).
Plant-derived Exosomes in Plant-Microbe Interactions
Plant-derived exosomes have been isolated from many plant species, including fruits, flowers, and vegetables, and play an important role in plant immune system regulation and defense responses.
Exosome-mediated transport is a critical mechanism for RNA secretion and delivery between plants and microorganisms. In co-evolution with interacting pathogens, plants have adapted exosome-mediated transboundary RNA interference (RNAi) for immune responses. For example, infection by fungal pathogens induces the expression of TET8 and TET9 in Arabidopsis thaliana.TET8 interacts with TET9 to form protein complexes and is enriched in the membranes of exosomes. These exosomes act as a defense by delivering small RNAs to the pathogen that mediate cross-species and cross-border RNAi to repress the expression of fungal virulence genes.
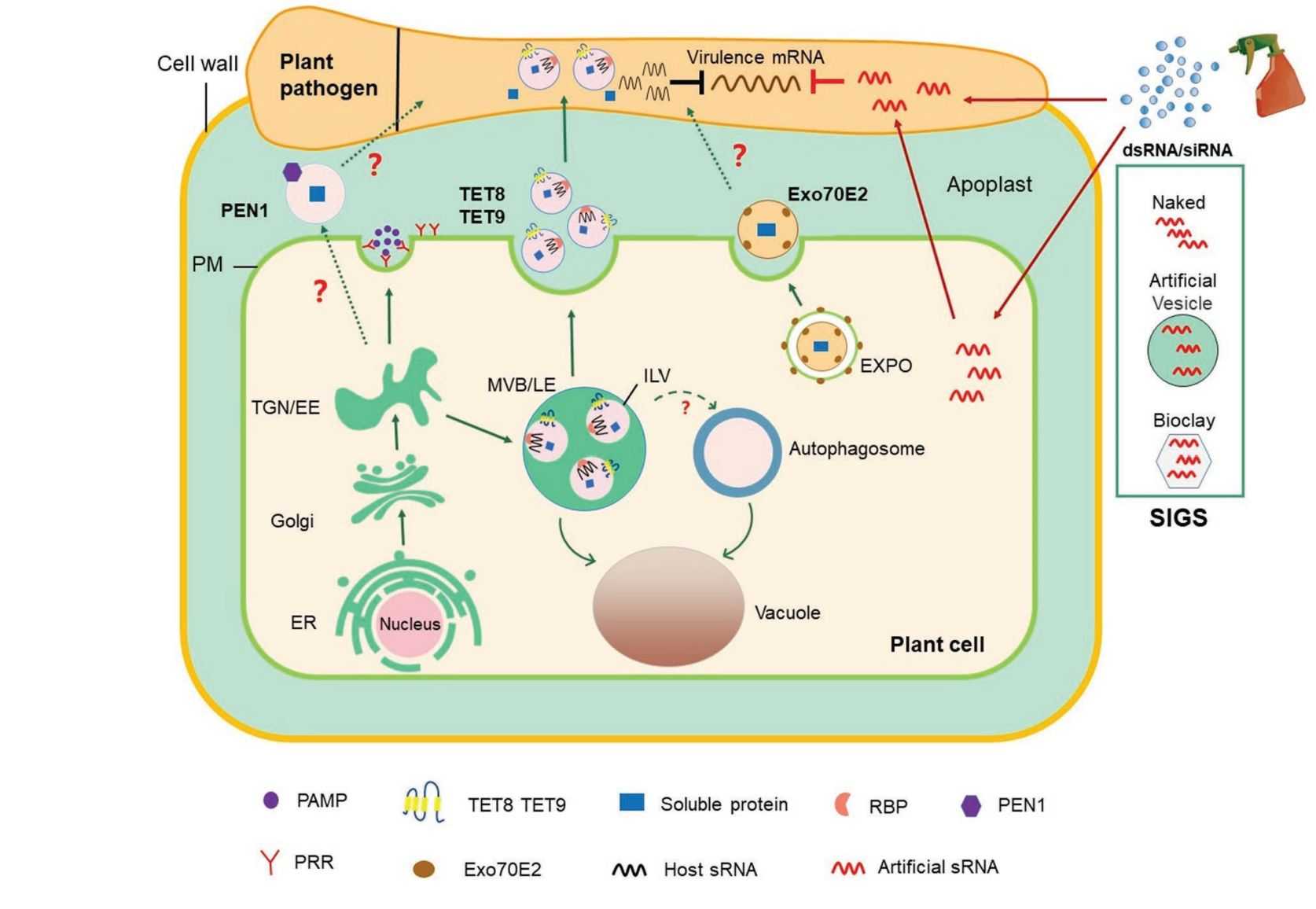 Figure 3. Role of exosome-mediated RNAi in plant-microbe interactions. (Liu G, et al., 2021)
Figure 3. Role of exosome-mediated RNAi in plant-microbe interactions. (Liu G, et al., 2021)
In addition to RNA cargo, plant-derived exosomes contain proteins involved in defense responses. Examples include BAK1-interacting receptor-like kinase 2, RPM1-interacting protein 4, glycine-rich protein 7, and BIR1-1 inhibitor. This suggests that these exosomes may regulate pathogen recognition by facilitating the extracellular transport of critical signaling proteins involved in immune signaling.
What is Transboundary RNAi?
sRNAs, including tiny RNAs (miRNAs) processed by Dicer-like (DCL) proteins from single-stranded stem-loop-forming RNA precursors and small interfering RNAs (siRNAs) processed by DCL proteins from double-stranded RNA (dsRNA) precursors, are loaded into AGO proteins to induce silencing of genes with complementary sequences. The plant host transports the sRNA into the fungal pathogen to repress the expression of virulence-related genes, thereby promoting a plant defense response. Analysis of sRNAs from fungal cells purified from infected plant tissues demonstrated the translocation of endogenous plant sRNAs to the fungus.
Novel Crop Protection Tools Based on Exosomes and RNAi
Host-induced gene silencing (HIGS) is an effective strategy for breeding resistant varieties. This method induces silencing of essential pathogen genes by expressing double-stranded RNA (dsRNA) targeting plant pathogen genes. However, HIGS also has the disadvantage of unstable expression. Therefore, researchers have developed a new technology, spray-induced gene silencing (SIGS), for controlling plant pathogens. The success of SIGS in plant disease management depends largely on the efficiency of RNA uptake by the pathogen. Studies have shown that exosomes protect sRNA from environmental degradation and are efficiently taken up by host cells. Therefore, using exosomes as RNAi carriers for crop protection is a promising area.
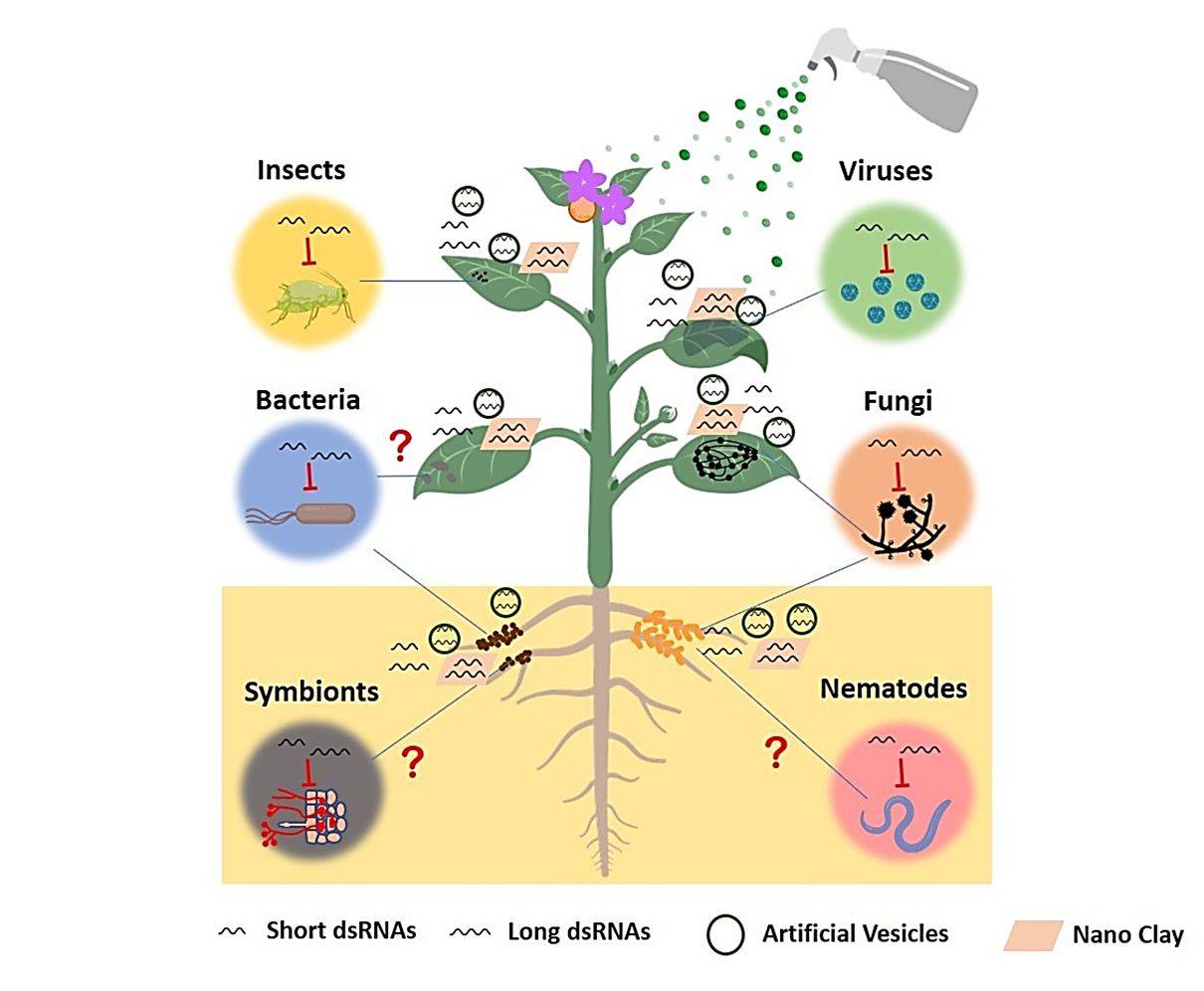 Figure 4. SIGS is an efficient disease control strategy in plants. (Cai Q, et al., 2019)
Figure 4. SIGS is an efficient disease control strategy in plants. (Cai Q, et al., 2019)
Our Products and Services
Studies have shown that exosomes play a critical role in transporting sRNAs from plant hosts to pathogens. The discovery of cross-species and cross-border RNAi and fungal RNA uptake has inspired researchers to design novel disease control strategies for agricultural pathogens and pests.
Creative Biostructure is a cutting-edge biotechnology company specializing in exosome services and exosome products. Our expertise lies in helping clients discover the vast potential of plant-derived exosomes for a variety of agricultural applications. With a deep understanding of exosome technology and its impact on agriculture, we provide innovative solutions and high-quality products to advance plant biology and crop development.
| Cat No. | Product Name | Source |
| Exo-PDELN01 | HQExo™ Exosome-Garlic | Exosome derived from Garlic |
| Exo-PDELN02 | HQExo™ Exosome-Ginger | Exosome derived from Ginger |
| Exo-PDELN03 | HQExo™ Exosome-Onion | Exosome derived from Onion |
| Exo-PDELN04 | HQExo™ Exosome-Potato | Exosome derived from Potato |
| PNE-VB13 | PNExo™ Exosome-Broccoli | Exosome derived from Broccoli |
| PNE-VE31 | PNExo™ Exosome-Enoki | Exosome derived from Enoki |
| PNE-VB14 | PNExo™ Exosome-Brussels Sprout | Exosome derived from Brussels Sprout |
| PNE-VC15 | PNExo™ Exosome-Cabbage | Exosome derived from Cabbage |
| PNE-FB13 | PNExo™ Exosome-Bilberry | Exosome derived from Bilberry |
| PNE-FA18 | PNExo™ Exosome-Apple | Exosome derived from Apple |
| PNE-FD50 | PNExo™ Exosome-Durian | Exosome derived from Durian |
| PNE-FG69 | PNExo™ Exosome-Green Plum | Exosome derived from Green Plum |
| PNE-FG84 | PNExo™ Exosome-Grape | Exosome derived from Grape |
| PNE-FC85 | PNExo™ Exosome-Concord Grape | Exosome derived from Concord Grape |
| PNE-FP93 | PNExo™ Exosome-Prickly Pear | Exosome derived from Prickly Pear |
| PNE-FL101 | PNExo™ Exosome-Lotus nut | Exosome derived from Lotus nut |
| PNE-FLA16 | PNExo™ Exosome-Aloe | Exosome derived from Aloe |
| PNE-FLA19 | PNExo™ Exosome-Azalea | Exosome derived from Azalea |
| PNE-FLM21 | PNExo™ Exosome-Morning glory | Exosome derived from Morning glory |
| PNE-FLC30 | PNExo™ Exosome-Carnation | Exosome derived from Carnation |
| PNE-FLA31 | PNExo™ Exosome-Amaryllis | Exosome derived from Amaryllis |
| PNE-FLC42 | PNExo™ Exosome-Begonia | Exosome derived from Begonia |
| PNE-FLA43 | PNExo™ Exosome-Anemone | Exosome derived from Anemone |
| PNE-FLD46 | PNExo™ Exosome-Dogwood | Exosome derived from Dogwood |
| PNE-FLD51 | PNExo™ Exosome-Daphne | Exosome derived from Daphne |
| Explore All Exosomes Isolated from Plants | ||
Please contact us for a formal quote. Partner with us to harness the power of plant-derived exosomes for sustainable agricultural practices and transformative results.
References
- Subha D, et al. Plant exosomes: nano conveyors of pathogen resistance. Discov Nano. 2023. 18(1): 146.
- Dad HA, et al. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol Ther. 2021. 29(1): 13-31.
- Liu G, et al. Extracellular Vesicles: Emerging Players in Plant Defense Against Pathogens. Front Plant Sci. 2021. 12: 757925.
- Cai Q, et al. Small RNAs and extracellular vesicles: New mechanisms of cross-species communication and innovative tools for disease control. PLoS Pathog. 2019. 15(12): e1008090.
- Chen Y-X, Cai Q. Plant Exosome-like Nanovesicles and Their Role in the Innovative Delivery of RNA Therapeutics. Biomedicines. 2023. 11(7): 1806.