Exosomes Target Delivery Based on Chemical Modification
Natural exosomes can be used as drug carriers to increase the effective concentration of drugs when used in tumor therapy, but there are sometimes problems such as short half-life and poor targeting. Generally, exosome surface modification can alleviate these limitations to some extent. In recent years, exosome surface proteins are usually attached to targeted proteins or peptides by chemical modification. Modified exosomes exhibit functionally better passive/active targeting, cellular uptake, and immune evasion than natural ones. In addition, specially modified exosomes can respond to exogenous stimuli, thereby allowing the release of carrier drugs.
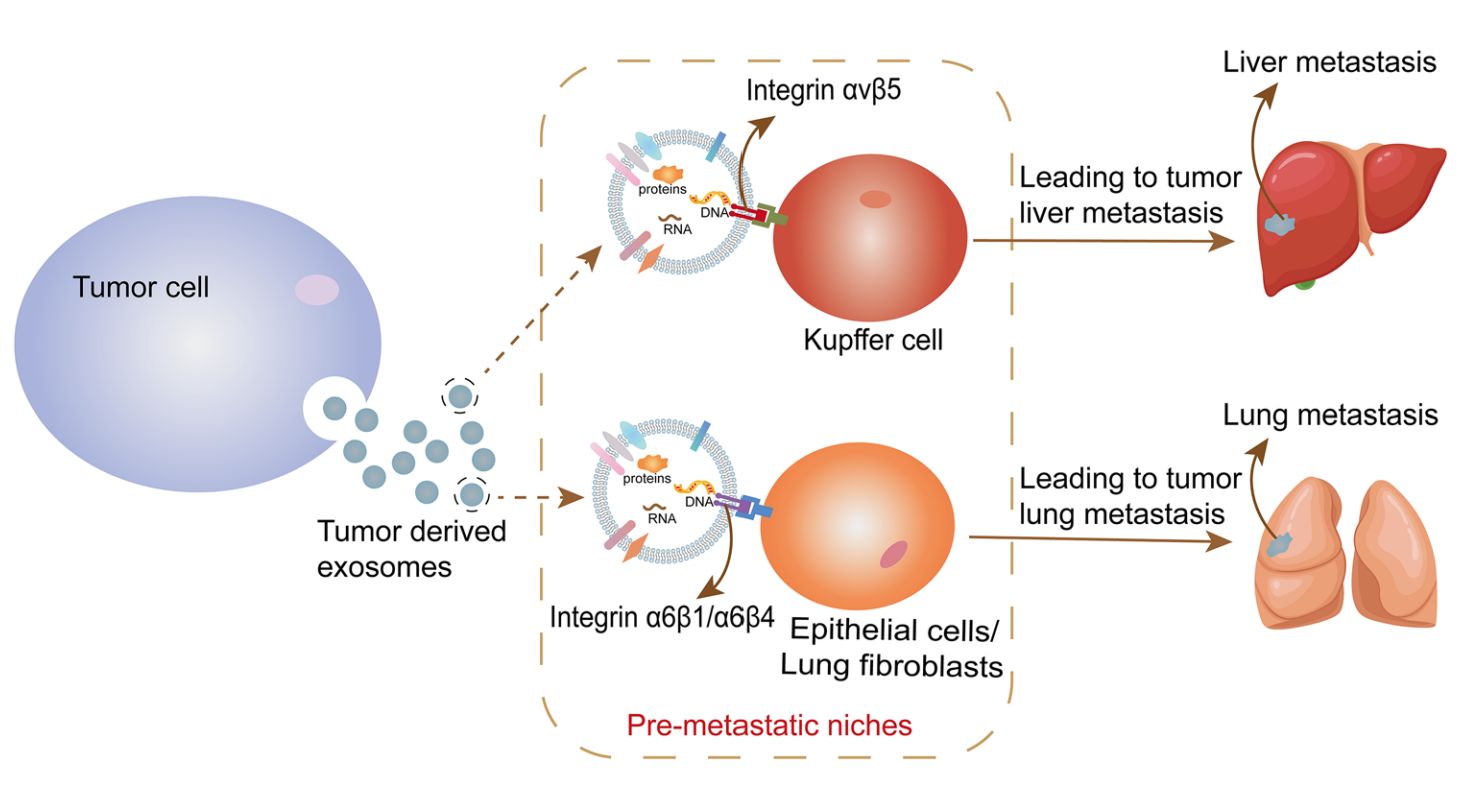 Figure 1. Exosomal membrane protein integrin induces its targeting metastasis. (He J, et al., 2022)
Figure 1. Exosomal membrane protein integrin induces its targeting metastasis. (He J, et al., 2022)
What is Chemical Modification?
Chemical modification refers to the attachment of target proteins to the surface of exosomes via lipid-binding proteins, membrane-binding proteins, or lipid-lipid interactions. These strategies are gentle and efficient but require tightly controlled reaction conditions.
- Covalent Modification
The most commonly used covalent modification method is click chemistry, which has been used to develop and prepare targeted nanoparticles. This method is a chemical coupling reaction that occurs under aqueous, buffered, and physiological conditions producing irreversible bonds. In contrast to conventional chemical reactions, click chemistry allows reactions to occur in aqueous solutions for short periods and the binding reactions do not affect exosome size or cellular uptake and absorption. Click chemistry utilizes covalent interactions between alkyne and azide residues to form stable triazole bonds that can be used to attach the targeted portion to the exosome surface in a variety of aqueous buffers such as water, alcohols, and dimethyl sulfoxide (DMSO). Based on this strategy, scientists have developed orthogonal chemical (click chemistry) targeting of exosomes.
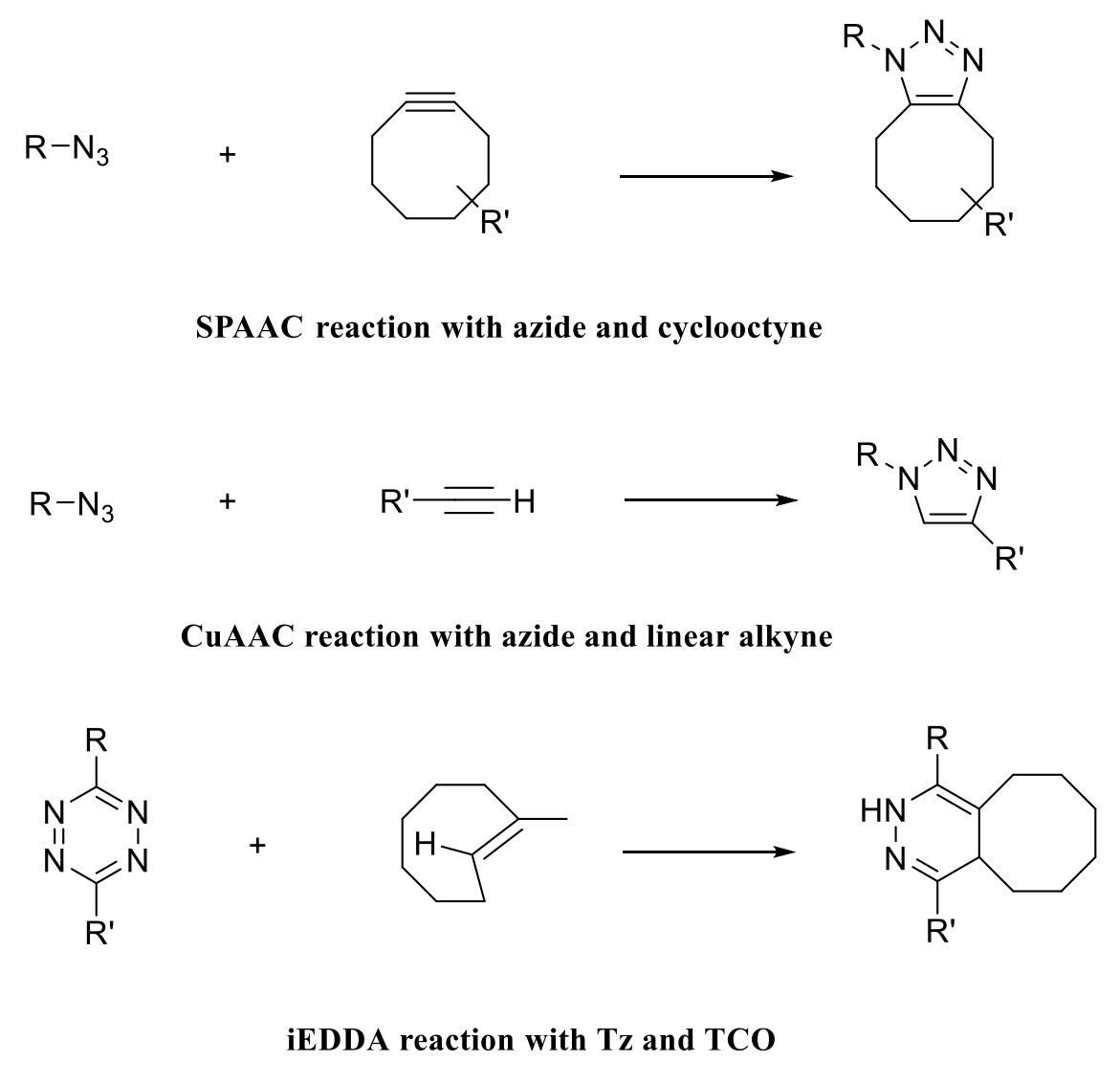 Figure 2. Schematics of various click chemistry reactions used to modify exosomes. (Akbari A, et al., 2022)
Figure 2. Schematics of various click chemistry reactions used to modify exosomes. (Akbari A, et al., 2022)
- Non-covalent Modification
It is also possible to employ non-covalent bonding, including charge and hydrophobic interactions. Compared to the covalent coupling, this strategy has relatively mild modification conditions but weak binding strength.
1) Multivalent electrostatic interactions - Covering the exosome membrane with a positive charge-transferring fraction, thereby increasing the targeting efficiency of the exosome to negatively charged biofilms.
2) Ligand-receptor interactions - Utilize natural receptors present on the exosome surface to attach targeting ligands.
3) Nucleic acid aptamer-based surface modification - The LZH8 aptamer is fused to two additional nucleotide sequences by hybridization chain reaction (HCR) to form a nano-assembly. These additional sequences enhance the binding affinity of the aptamer to the exosome surface.
4) CP05 peptide anchor modification- The CP05 peptide has a strong affinity for the second EC loop of CD63, making it a good link between the targeting/therapeutic portion and the exosome surface. The CP05 anchor modification has been shown to preserve the natural size and morphological features of the exosome without affecting its distribution in vivo.
Examples of Chemical Modification to Target Exosomes
Targeted delivery of drugs to therapeutically active sites with low immunogenicity and systemic toxicity is essential for tumor therapy. Exosomes, as nanoscale membrane vesicles of natural origin, are engineered with chimeric peptides for plasma membrane- and nucleus-targeted photosensitizer delivery and synergistic photodynamic therapy (PDT). The research found that plasma membrane-targeted PDT of chimeric peptide-engineered exosomes (ChiP-Exo) directly disrupts membrane integrity and leads to a degree of cell death and that ChiP-Exo in the nucleus activates reactive oxygen species (ROS) in situ, resulting in potent and synergistic photodynamic therapy. This strategy minimizes systemic toxicity and significantly improves the therapeutic effect of tumor growth inhibition, providing a new idea for the development of personalized biomedicine to achieve precision tumor therapy.
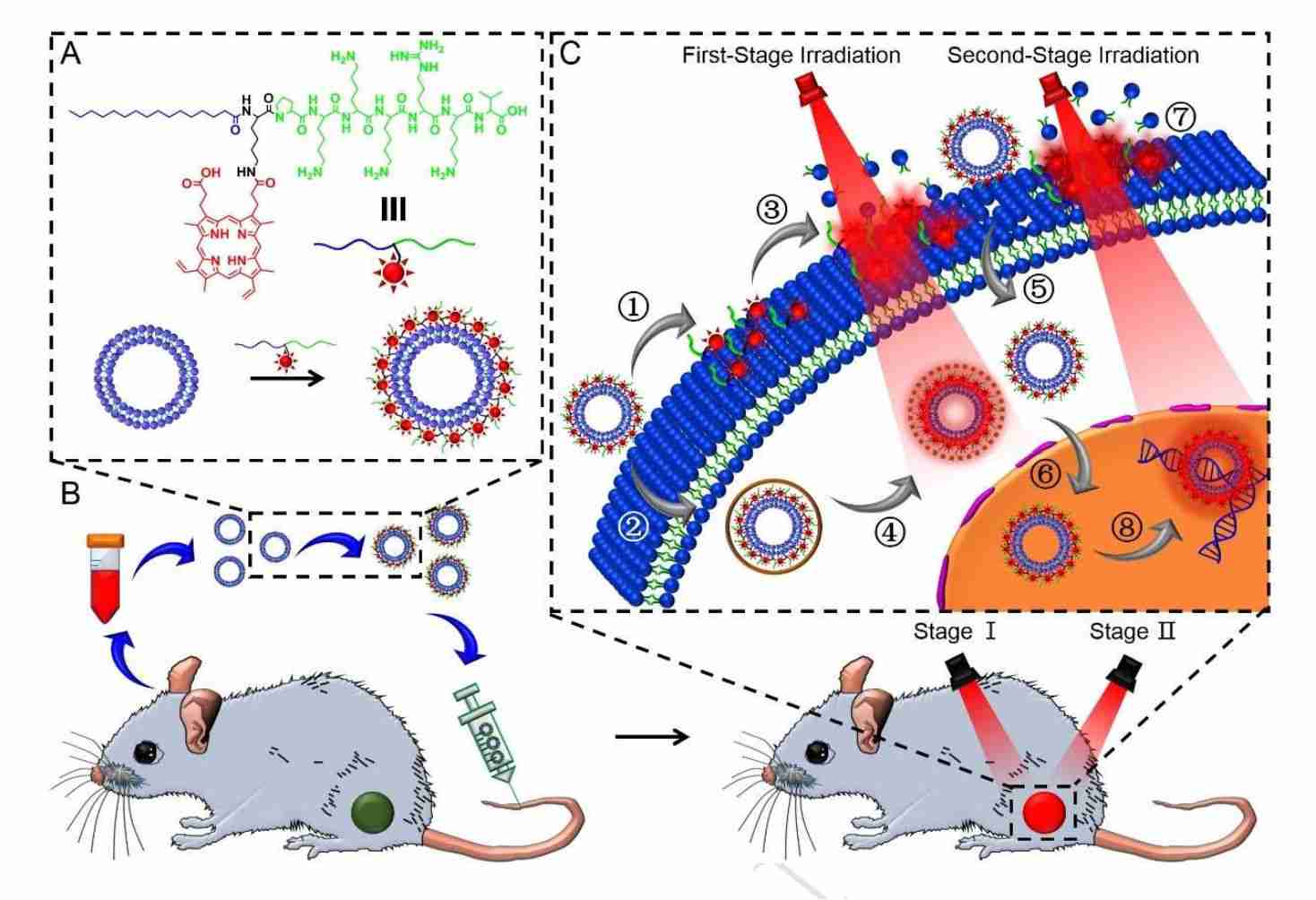 Figure 3. Schematic of ChiP-Exo for dual-stage light-guided plasma membrane and nucleus-targeted PDT. (Cheng H, et al., 2019)
Figure 3. Schematic of ChiP-Exo for dual-stage light-guided plasma membrane and nucleus-targeted PDT. (Cheng H, et al., 2019)
Immunotherapy has received increasing attention in the treatment of pancreatic ductal adenocarcinoma (PDAC). However, due to the unique tumor microenvironment and low cancer immunogenicity of PDAC, developing new therapies remains a great difficulty. In recent years, researchers have proposed an exosome-based dual-delivery biosystem for enhancing PDAC immunotherapy and disrupting tumor immunosuppression. The delivery system consists of bone marrow mesenchymal stem cell (BM-MSC) exosomes, electroporation-loaded galectin-9 siRNA, and surface modification with oxaliplatin (OXA) prodrug as an immunogenic cell death (ICD) trigger. The results showed that BM-MSC exosomes significantly improved tumor targeting and thus increased drug accumulation at the tumor site.
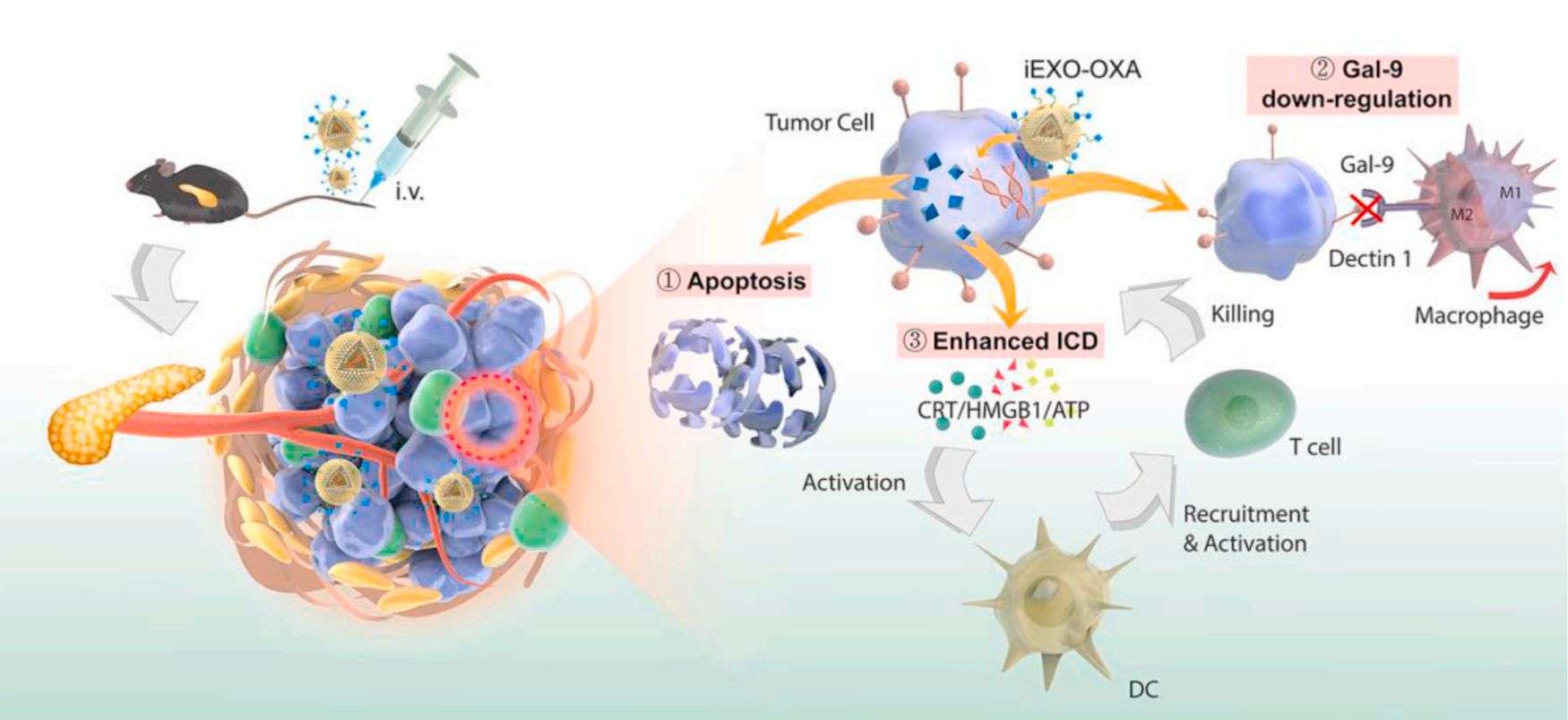 Figure 4. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. (Zhou W, et al., 2021)
Figure 4. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. (Zhou W, et al., 2021)
Our Products
As a leader in exosome research, Creative Biostructure provides aptamer-based exosome targeting services designed to help clients improve the targeted delivery of exosomes.
In addition, we have a range of high-quality exosome products for exploring the potential applications of exosomes in drug delivery.
| Cat No. | Product Name | Source |
| Exo-CH15 | HQExo™ Exosome-BLCL21 | Exosome derived from EBV transformed lymphoblastoid B cells (BLCL21 cell line) |
| Exo-CH18 | HQExo™ Exosome-LnCAP | Exosome derived from human prostate adenocarcinoma (LnCAP cell line) |
| Exo-HDBF-01 | HQExo™ Exosome-SDH-Alzheimer's plasma | Exosome derived from Single Donor Human Alzheimer's plasma |
| Exo-HDBF-03 | HQExo™ Exosome-SDH-Atopic Dermatitis plasma | Exosome derived from Single Donor Human Atopic Dermatitis plasma |
| Exo-IC01 | HQExo™ Exosome-BC3 | Exosome derived from human B lymphocyte cell line (BC-3 ) |
| Exo-IC03 | HQExo™ Exosome-Jurkat E6-1 | Exosome derived from human T lymphocyte cell line (Jurkat E6-1) |
| PNE-FA95 | PNExo™ Exosome-Annona Squamosa | Exosome derived from Annona Squamosa |
| PNE-FLB67 | PNExo™ Exosome-Bamboo | Exosome derived from Bamboo |
| Exo-SC03 | HQExo™ Exosome-hTERT | Exosome derived from hTERT-immortalized Mesenchymal Stem Cell |
| Exo-SC02-2 | HQExo™ Exosome-Pla-MSC | Exosome derived from human placental derived mesenchymal stem cell |
| Exo-EV-A-001 | HQExo™ Exosome-Bovine Plasma exosome | Exosome derived from Bovine Plasma |
| Exo-BF01 | HQExo™ Exosome-CSF | Exosome derived from human pooled CSF (healthy donors) |
| Explore All Exosomes Products | ||
If you are interested in our exosome targeting service, please feel free to contact us. We look forward to working with you!
References
- He J, et al. Exosomal targeting and its potential clinical application. Drug Deliv Transl Res. 2022. 12(10): 2385-2402.
- Akbari A, et al. Engineered Exosomes for Tumor-Targeted Drug Delivery: A Focus on Genetic and Chemical Functionalization. Pharmaceutics. 2022. 15(1): 66.
- Cheng H, et al. Chimeric peptide engineered exosomes for dual-stage light guided plasma membrane and nucleus targeted photodynamic therapy. Biomaterials. 2019. 211: 14-24.
- Zhou W, et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials. 2021. 268: 120546.
- Choi H, et al. Biodistribution of Exosomes and Engineering Strategies for Targeted Delivery of Therapeutic Exosomes. Tissue Eng Regen Med. 2021. 18(4): 499-511.