MemPro™ EPHA1
Creative Biostructure provides custom MemPro™ gene-to-structure services for EPHA1.
Erythropoietin-producing hepatocellular receptor (EPH receptor) is a receptor tyrosine kinase (RTK) that plays a key role in developmental processes, including guidance of the migration of axons and cells in the nervous system. In adult organism, Eph-ephrin interactions can also trigger a wide array of cellular responses, including cell boundary formation, motility, adhesion, and repulsion, especially for neuronal and endothelial cells, whereas deregulated reemergence of Eph function appears to contribute to mechanism of tissue injury and of tumor invasion and metastasis. There are some evidence suggests that Eph-ephrin interactions may have a role in synaptic plasticity, learning, memory formation and metal disease.
Depend on the ligands ephrin A and B, receptors are divided into two classes. Ephrin-A ligands share a membrane-tethered glycosylphosphatidylinositol anchor, whereas ephrin-B ligands have a transmembrane domain and a short cytoplasmic tail. EPHA1, similar to other RTK family member, contains a single transmembrane (TM) helical domain with an N-terminal glycosylated extracellular region comprised of a ligand-binding domain with immunoglobulin-like motifs, a cysteine-rich region with an epidermal growth factor-like motif, and two fibronectin type III repeats.
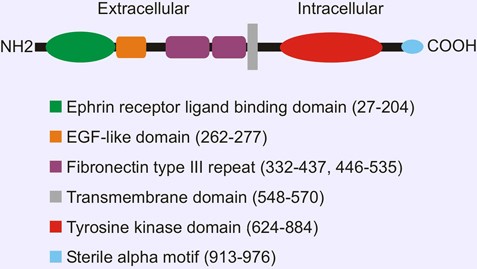
Figure 1. Domain organization of EPHA1
The receptor tyrosine kinases conduct biochemical signals across plasma membrane via lateral dimerization in which their transmembrane domains play an important role. Structures of EphA1 TM helix dimers in phospholipid bicelles have been determined using NMR. EphA1 TM segments associate in a right-handed parallel α-helical bundle interacting via an N-terminal glycine zipper motif.
The ephrin receptors and their ligands, the ephrins are frequently overexpressed in a wide variety of cancers, including breast, small cell-lung and gastrointestinal cancers, melanomas and neuroblastomas. Since EPHA1 interacts with actin cytoskeleton and provides a novel mechanism for achieving highly localized regulation of growth cone motility, blocking of EPHA class receptor activation appears to inhibit angiogenesis in some types of tumor. However, EphA1 over-expression was more prevalent in stage II compared to stage III colorectal cancer (CRC) and low EphA1 expression significantly correlated with poor survival. Epigenetic silencing appeared to explain the loss of EphA1 expression as methylation of the EphA1 CpG Island strongly correlated with low EphA1 expression. EphA1 could be a potential prognostic marker in CRC. Although therapies targeting high EphA1 expression seem plausible in CRC, the loss of expression in advanced disease suggests a potential risk that targeted therapy, by selecting for loss of expression, might contribute to disease progression.
References:
Bocharov E V, Mayzel M L, Volynsky P E, et al. Spatial structure and pH-dependent conformational diversity of dimeric transmembrane domain of the receptor tyrosine kinase EphA1[J]. Journal of Biological Chemistry, 2008, 283(43): 29385-29395.
Chavent M, Chetwynd A P, Stansfeld P J, et al. Dimerization of the EphA1 receptor tyrosine kinase transmembrane domain: Insights into the mechanism of receptor activation[J]. Biochemistry, 2014, 53(42): 6641-6652.
Nievergall E, Saunders T, Lackmann M. Targeting of EPH receptor tyrosine kinases for anticancer therapy[J]. Critical Reviews™ in Oncogenesis, 2012, 17(2).