Structural Research of Phospholipid Transferases
Phospholipid transferase participates in the transfer of phospholipids between different membranes and plays a crucial role in maintaining the lipid composition and asymmetry of the membrane. It participates in physiological activities such as vesicle transport, lipid signaling, lipid homeostasis, lipid transport, cell apoptosis, and coagulation.
Research progress in phospholipid transferases
In past research, significant progress has been made in the structural analysis of phospholipid transferases. X-ray diffraction has become a powerful tool for determining the high-resolution structure of these molecules. Multiple studies have reported the cryoelectron microscopy structures of phospholipid transferases, including phospholipid transfer protein (PLTP), phosphatidylinositol transfer protein (PITP), phosphatidylcholine transfer protein (PCTP), phospholipid transfer protein oxygen sterol binding protein (OSBP), and phosphatidylserine transfer protein (PSTP).
Research progress in PLTP
PLTP, as a typical phospholipid transferase, mediates phospholipid transport between lipoproteins and is crucial for the treatment of lipoprotein abnormalities and cardiovascular diseases. The researchers used various microscopic techniques to show the structure of PLTP. It can be seen to have a banana-shaped structure containing long narrow channels connecting two distal openings and two lipid-binding pockets. Insight into the interaction of PLTP with lipoproteins at the molecular level provides a basis for understanding the mechanism of PLTP-dependent lipid transfer for the treatment of dyslipidemia.
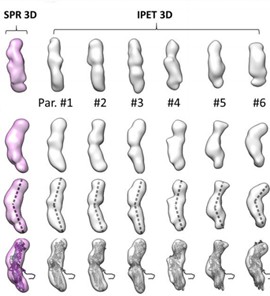 Figure 1. 3D reconstruction of PLTP particles. (Zhang M, et al., 2018)
Figure 1. 3D reconstruction of PLTP particles. (Zhang M, et al., 2018)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| PITP-Beta complexed to phosphatidylcholine | Rattus norvegicus | X-ray diffraction | 2.18 Å | 2A1L |
| PITP alpha void of bound phospholipid | Mus musculus | X-ray diffraction | 2 Å | 1KCM |
| PITP complexed to phosphatidylcholine | Rattus norvegicus | X-ray diffraction | 2.2 Å | 1T27 |
| PITP-alpha complexed to phosphatidylinositol | Homo sapiens | X-ray diffraction | 2.9 Å | 1UW5 |
| Phosphatidylinositol transfer protein SEC14P | Saccharomyces cerevisiae | X-ray diffraction | 2.5 Å | 1AUA |
| Sfh2 in an apo form | Saccharomyces cerevisiae S288C | X-ray diffraction | 2.2 Å | 7WWE |
| Sfh3 | Saccharomyces cerevisiae S288C | X-ray diffraction | 2 Å | 4J7P |
| Sfh5 | Saccharomyces cerevisiae YJM789 | X-ray diffraction | 2.9 Å | 6W32 |
| Sfh2 complexed with phosphatidylinositol | Saccharomyces cerevisiae S288C | X-ray diffraction | 1.5 Å | 7WVT |
Table 1. Structural research of phospholipid transferases.
In recent years, several breakthroughs and discoveries have been made in the research of phospholipid transferases using various structural biology methods and techniques, including NMR spectroscopy, cryo-electron microscopy (cryo-EM) and X-ray crystallography. The studies can reveal the relationship between structure and function of phospholipid transferases.
Creative Biostructure is a research and service company specializing in protein structural biology. Our professional team has rich experience and expertise to provide full support and solutions for clients' research. We will provide high-quality data and analysis results according to clients' needs and research objectives, help gain a deeper understanding of the structure and function of phospholipid transferases, and promote clients' research to achieve breakthroughs. If you are interested in our services, please contact us for a more detailed description.
Reference
- Zhang M, et al. Structural basis of the lipid transfer mechanism of phospholipid transfer protein (PLTP). Biochim Biophys Acta Mol Cell Biol Lipids. 2018.1863(9):1082-1094.