Structural Research of Type II Secretion Systems
The type II secretion system (T2SS) common to Gram-negative bacteria is a macromolecular complex that spans the cell membrane. It allows substrates to cross the cell membrane and substrates released into the environment act as effectors to promote biofilm formation, nutrient acquisition, and pathogenicity. In animals and plants, T2SS is considered a critical driver of virulence. In recent years, advances in cryo-electron microscopy have allowed the structure of T2SS to be resolved, revealing its unique action mechanism.
Structural components of the T2SS secretion system
Recently, researchers have elucidated unique structural features that add essential value to the requirement of functional and structural data for research on the T2SS secretion system. In the T2SS, several major regions are included. Among them, the outer membrane region consists of an outer membrane-embedded core (e.g., GspD or PulD) composed of the major trypticin channels. The pilotin region is located in the periplasm and faces the outer membrane (OM) (e.g., GspS, AspS, or PulS). The assembly platform consists of multiple subunits (e.g., PulC, PulE, PulL, PulM, or PulN) positioned in the underlying periplasmic/peptidoglycan region and extends to the inner membrane (IM). The pseudopilus extrusion machinery consists of helical filaments that propel the fully folded matrix through the trypticin channel.
Structure of the T2SS core
The T2SS fromD. radiodurans R1 is isolated and resolved at 2 Å. The core consists of trypsin (a pentadecameric GspD) facing the inner membrane. The GspD outer membrane region has a dominant 60 β-fold β-barrel structure. For each trypticin monomer, two protrusions appear, an external protrusion in the OM (EUP) and a broader external bottom protrusion in the periplasm (EBP). Internally, the Beta hairpin basal sequence constricts the channel and forms the periplasmic gate. Below the OMR, the periplasmic region contains the N structural domain, N3, N2, N1 and N0. Among them, The N0 structural domain connects to the N-terminal portion of the GspD, the IMRR (inner membrane-associated region), and recognizes the N structural domain.
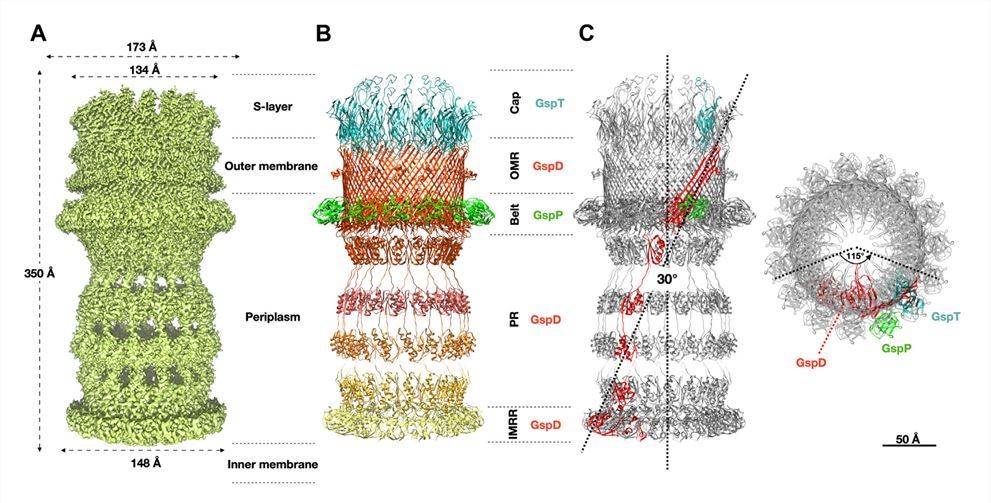 Figure 1. The T2SS structure of D. radiodurans. (Farci D, et al., 2024)
Figure 1. The T2SS structure of D. radiodurans. (Farci D, et al., 2024)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| cyto-EpsL: the cytoplasmic domain of EpsL, an inner membrane component of the type II secretion system | Vibrio cholerae | X-ray diffraction | 2.7 Å | 1W97 |
| ATPase GspE in complex with the cytoplasmic domain of GspL from the type II Secretion system | Vibrio vulnificus CMCP6 | X-ray diffraction | 2.83 Å | 4PHT |
| Pullulanase-specific type II secretion system integral cytoplasmic membrane protein GspL (C-terminal fragment; residues 309-397) | Klebsiella pneumoniae subsp. pneumoniae NTUH-K2044 | X-ray diffraction | 1.93 Å | 5HL8 |
| Periplasmic domain of XcpY, tI crystal form | Pseudomonas aeruginosa PAO1 | X-ray diffraction | 2.501 Å | 5N7L |
| Periplasmic domain of XcpY, oP crystal form | Pseudomonas aeruginosa | X-ray diffraction | 2 Å | 6GHU |
| PulL C-ter domain | Klebsiella oxytoca | X-ray diffraction | 1.895 Å | 8A9W |
| PulM C-ter domain | Klebsiella oxytoca | X-ray diffraction | 1.523 Å | 8A9X |
| PulL-PulM C-terminal domain heterocomplex | Klebsiella oxytoca | X-ray diffraction | 2.77 Å | 8AB1 |
| PulM C-terminal domain | Klebsiella oxytoca | SOLUTION NMR | / | 7ZE0 |
| ETEC Pilotin-Secretin AspS-GspD complex | Escherichia coli ETEC H10407 | Cryo-EM single particle analysis | 3.2 Å | 5ZDH |
| Minor pseudopilin binary complex of XcpV and XcpW from the Type 2 secretion system | Pseudomonas aeruginosa PAO1 | X-ray diffraction | 2 Å | 5BW0 |
| Minor pseudopilin ternary complex of XcpVWX from the Type 2 secretion system | Pseudomonas aeruginosa PAO1 | X-ray diffraction | 2.041 Å | 5VTM |
| Minor pseudopilin ternary complex of XcpVWX from the Type 2 secretion system in the P3 space group | Pseudomonas aeruginosa PAO1 | X-ray diffraction | 2.85 Å | 6UTU |
| N0 domain of the type II secretin | Escherichia coli ETEC H10407 | X-ray diffraction | 1.43 Å | 4JTM |
| Pilotin/secretin peptide Complex | Dickeya dadantii 3937 | X-ray diffraction | 2.15 Å | 4K0U |
| XcpQN012 in complex with VHH04 | Lama glama | X-ray diffraction | 2.9 Å | 5MP2 |
| XcpQN012 | Pseudomonas aeruginosa | X-ray diffraction | 2.98 Å | 5NGI |
| T2SS secretin XcpQ | Pseudomonas aeruginosa PAO1 | Cryo-EM single particle analysis | 3.57 Å | 5WLN |
| Type II secretion system secretin GspD | Vibrio cholerae O1 biovar El Tor str. N16961 | Cryo-EM single particle analysis | 3.26 Å | 5WQ8 |
| Type II secretion system secretin GspD G453A mutant | Vibrio cholerae O1 biovar El Tor str. N16961 | Cryo-EM single particle analysis | 4.22 Å | 5WQ9 |
| Type II secretion system outer membrane complex. PulD, PulS, and PulC HR domain. | Klebsiella pneumoniae | Cryo-EM single particle analysis | 4.3 Å | 6HCG |
| ExeD | Aeromonas hydrophila | Cryo-EM single particle analysis | 3.7 Å | 6I1X |
| Cytoplasmic domains of EpsF from the Type 2 Secretion System | Vibrio cholerae | X-ray diffraction | 1.9 Å | 2VMA |
| Cytoplasmic domains of EpsF from the Type 2 Secretion System | Vibrio cholerae | X-ray diffraction | 1.7 Å | 3C1Q |
Table 1. Structural research of the type II secretion systems.
Structural analysis is essential for research on proteins and other biomolecules. Creative Biostructure is a leading provider of such services, and our team of experts uses a variety of cutting-edge techniques to provide clients with high-quality structural information, such as X-ray crystallography, cryo-electron microscopy (cryo-EM), and NMR spectroscopy.
We have a team of experienced structural biologists dedicated to delivering high-quality analyses. We work closely with our clients to provide comprehensive reports. If you are researching protein structure and function in the field of type II secretion systems, we are the ideal partner for you! For more information, please contact us.
References
- Farci D, et al. Structural characterization and functional insights into the Type II Secretion System of the poly-extremophile Deinococcus radiodurans. J Biol Chem. 2024. 300(2): 105537.
- Naskar S, et al. The structure and mechanism of the bacterial type II secretion system. Mol Microbiol. 2021. 115(3): 412-424.
- Howard SP, et al. Structure and assembly of pilotin-dependent and -independent secretins of the type II secretion system. PLoS Pathog. 2019. 15(5): e1007731.