Structural Research of Very Long Chain Fatty Acid Elongases
The very long-chain fatty acid elongase (ELOVL) family plays an essential role in lipid metabolism and cellular function, catalyzing the first step in the fatty acid elongation cycle. ELOVL synthesizes very long-chain fatty acids (VLCFA) by promoting the elongation of fatty acids beyond typical chain lengths. VLCFA is an essential component of a variety of cellular processes, including lipid metabolism, membrane structure, and cellular signaling. Research has linked the dysregulation of ELOVL expression to a variety of diseases, including metabolic disorders, neurodegenerative diseases, skin diseases, and cancer. The identification of ELOVL-based biomarkers may advance diagnostics and personalized medicine.
Structural and functional overview of ELOVL
Members of the ELOVL family share common structural and functional characteristics. These enzymes are integral membrane proteins present in the endoplasmic reticulum (ER) that act as catalysts in the fatty acid elongation process. In structure, ELOVL consists of a transmembrane structural domain that anchors it to the endoplasmic reticulum membrane and a catalytic structural domain that facilitates the enzymatic reaction. The catalytic structural domain typically contains conserved motifs and residues essential for substrate binding and enzymatic activity. Functionally, ELOVL works by adding two carbon units to the acyl chain, allowing for the gradual addition of carbon atoms to the growing fatty acid chain, ultimately producing VLCFA.
Structural analysis of human ELOVL7
There are seven mammalian ELOVLs (ELOVL1-7), and each elongase has different preferences for acyl chain length and unsaturation. To understand the molecular basis of acyl chain lengthening by ELOVL, researchers have resolved the structure of human ELOVL7 by X-ray crystallography. Overall, ELOVL7 has seven transmembrane helices (TM1-TM7), with TM2-7 forming six TM inverted barrels around a narrow tunnel. The two 3-helix units are assembled in an inverted repeating fashion around the central tunnel, with TM1 located outside the barrel against TM3/4. The central tunnel has a narrow opening in the cytoplasmic plane, which is sealed at the end of its ER lumen by a short-buried loop between TMs4-5, which connects the two halves of the barrel.
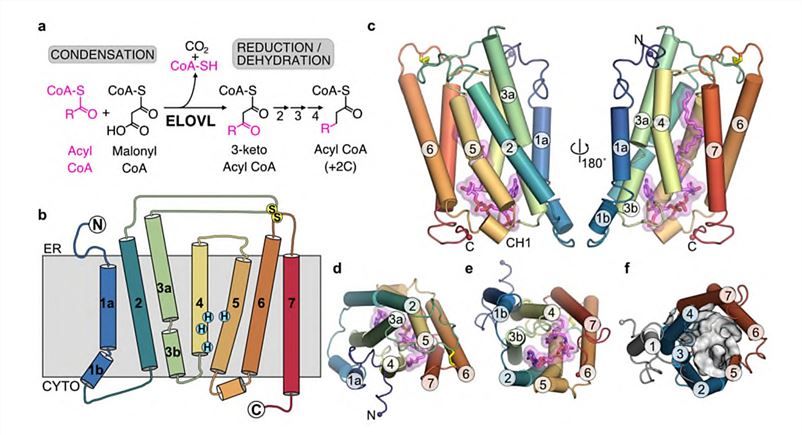 Figure 1. The overall structure of ELOVL7. (Nie L, et al., 2021)
Figure 1. The overall structure of ELOVL7. (Nie L, et al., 2021)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| ELOVL fatty acid elongase 7 (ELOVL7) | Homo sapiens | X-ray diffraction | 2.052 Å | 6Y7F |
Table 1. Structural research of the very long chain fatty acid elongases.
As a pioneer in structural biology research, Creative Biostructure provides cutting-edge services to satisfy the requirements of researchers and pharmaceutical companies worldwide. Our structural analysis services provide a detailed understanding of the structure of a wide range of biomolecules, including very long-chain fatty acid elongases.
Using advanced techniques such as X-ray crystallography, cryo-electron microscopy (cryo-EM), and NMR spectroscopy, our team of experts can elucidate the three-dimensional structures of very long chain fatty acid elongases, helping to unravel the mechanisms of their function and research on them as therapeutic targets for anti-cancer treatments. Please contact us to explore the potential of our services to enhance your research efforts and move you closer to achieving your scientific goals.
References
- Nie L, et al. The structural basis of fatty acid elongation by the ELOVL elongases. Nat Struct Mol Biol. 2021. 28(6): 512-520.
- Wang X, et al. A comprehensive review of the family of very-long-chain fatty acid elongases: structure, function, and implications in physiology and pathology. Eur J Med Res. 2023. 28(1): 532.
