Protein Phosphorylation
Protein phosphorylation, a reversible post-translational modification, is critical in cellular signal transduction and regulation. This modification, characterized by the attachment of phosphate groups to specific amino acids, predominantly serine, threonine, and tyrosine, has profound implications for protein structure, function, and interaction networks. Structural biology provides essential insights into understanding how phosphorylation modulates protein conformations, binding affinity, and catalytic activity. Some structural biology techniques are used to study phosphorylation, including X-ray crystallography, Nuclear Magnetic Resonance (NMR) spectroscopy, and Cryo-Electron Microscopy (cryo-EM).
Overview of Protein Phosphorylation
Protein phosphorylation is an important process that helps regulate many key cellular functions. It plays a role in metabolism, growth, and cell death. It works by adding a phosphate group from ATP to specific amino acids in a protein. This change can affect the protein's behavior in different ways. It can influence protein's stability, interaction potential, and catalytic activity. The dynamic nature of phosphorylation makes it crucial in processes such as cell signaling cascades. Misregulation of protein phosphorylation is associated with several diseases, including cancer, neurodegeneration, and cardiovascular disorders.
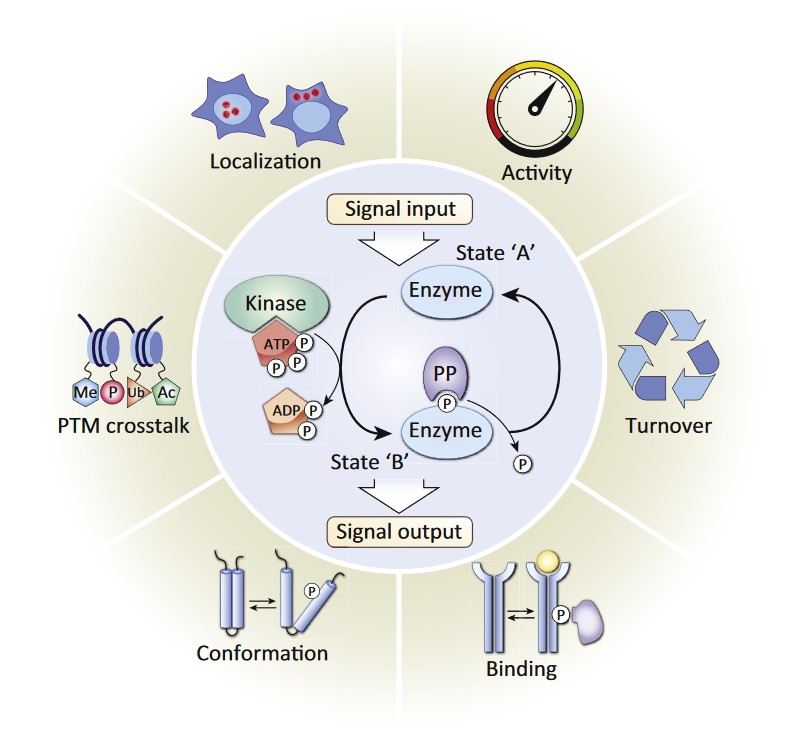 Figure 1: Reversible Protein Phosphorylation Is a Molecular Switch Mechanism. Reversible protein phosphorylation is characterized by the addition of phosphate donated from ATP and the removal of phosphate from a phosphorylated protein substrate, catalyzed by protein kinase and phosphatase (PP) enzymes respectively (center circle). This pervasive post-translational modification (PTM) serves as molecular switch mechanism, modulating diverse protein functions including enzymatic activity, protein turnover, interactions, conformation, localization, and crosstalk with other PTMs, which in turn regulate broad cellular biological functions. Kinases and their substrates form vast networks of dynamic protein phosphorylation within cells. (Humphrey SJ et al., 2015)
Figure 1: Reversible Protein Phosphorylation Is a Molecular Switch Mechanism. Reversible protein phosphorylation is characterized by the addition of phosphate donated from ATP and the removal of phosphate from a phosphorylated protein substrate, catalyzed by protein kinase and phosphatase (PP) enzymes respectively (center circle). This pervasive post-translational modification (PTM) serves as molecular switch mechanism, modulating diverse protein functions including enzymatic activity, protein turnover, interactions, conformation, localization, and crosstalk with other PTMs, which in turn regulate broad cellular biological functions. Kinases and their substrates form vast networks of dynamic protein phosphorylation within cells. (Humphrey SJ et al., 2015)
Mechanisms of Protein Phosphorylation
Protein phosphorylation is catalyzed by specific enzymes called protein kinases. These enzymes have the ability to recognize specific target residues in proteins. They transfer a phosphate group from ATP to the hydroxyl group of specific amino acid residues, which can be serine, threonine, or tyrosine. The phosphorylation process modifies the amino acid side chain by introducing a negatively charged phosphate group. This modification generates both steric and electrostatic effects that can significantly alter protein structure and function.
On the other hand, protein phosphatases remove the phosphate groups from proteins. Their action makes phosphorylation a reversible modification, which is crucial for cellular regulation. The delicate interplay between kinases and phosphatases ensures precise control over phosphorylation levels within cells. This dynamic mechanism allows for rapid and flexible response to cellular signals and environmental changes.
Structural Impacts of Phosphorylation
Phosphorylation induces changes in protein structure, which can affect protein function in several ways:
- Conformational Changes: Protein phosphorylation creates new hydrogen bonds or repulsive forces that lead to significant conformational changes. These changes often alter the active or binding sites of the protein, modulating its interactions with other molecules. In kinases, phosphorylation of the activation loop is required for structural rearrangements that switch the enzyme from an inactive to an active state, facilitating substrate binding and catalysis.
- Alteration of Surface Properties: The addition of negatively charged phosphate groups affects the surface electrostatics of proteins, influencing protein-protein interactions. In some cases, phosphorylation promotes binding by creating new interaction surfaces, while in others, it induces repulsion between molecules, inhibiting interactions.
- Allosteric Regulation: Phosphorylation can exert allosteric effects, wherein modification at one site triggers structural rearrangements at a remote functional site. Such allosteric regulation by phosphorylation is common in metabolic enzymes, allowing them to respond to cellular signals and regulate metabolic flux.
Functional Roles of Protein Phosphorylation
Protein phosphorylation serves various functions in cellular signaling and regulation:
- Signal Transduction: Phosphorylation is the basis of signal transduction, and particularly receptor tyrosine kinase (RTK) signaling. When ligands bind to RTKs, tyrosine residues on the receptor become phosphorylated, allowing them to form binding sites for downstream signaling molecules.
- Cell Cycle Control: By regulating the cyclins and CDKs that drive cell cycle progression, phosphorylation regulates cell cycle progression. For example, CDK1 phosphorylation stimulates its kinase and triggers the cell cycle transition from G2 to M. Structural analysis of CDKs has demonstrated how phosphorylation-induced conformational changes activate these enzymes at specific cell cycle checkpoints.
- Metabolic Regulation: Phosphorylation regulates enzymes that function in metabolism, such as glycogen phosphorylase, whose activation is regulated by phosphorylation. Molecular analyses of glycogen phosphorylase have revealed how phosphorylation-induced modifications to its quaternary structure enable it to interact with glycogen, thus controlling glycogenolysis.
Techniques for Studying Phosphorylation
Structural biology techniques have been instrumental in examining how phosphorylation affects protein structure and function. Key techniques are summarized in the table below. These techniques have revealed insights into the structural dynamics of protein phosphorylation and the role of specific residues and domains in mediating its effects.
| Experimental Techniques | Attribute measured |
| X-ray Crystallography | This technique provides high-resolution structural data on phosphorylated proteins, allowing precise analysis of changes in protein conformation upon phosphorylation. However, crystallography requires proteins to form crystals, which may not always retain the dynamic conformations associated with phosphorylation. |
| Nuclear Magnetic Resonance (NMR) Spectroscopy | NMR allows the observation of proteins in solution, preserving their natural flexibility. This technique is particularly valuable for studying transient phosphorylation events and dynamic structural rearrangements. |
| Cryo-Electron Microscopy (cryo-EM) | Cryo-EM has advanced significantly, providing structural data for large, complex, and sometimes dynamic proteins. It has been valuable for capturing phosphorylated states of proteins that undergo significant conformational changes and for studying large multi-protein complexes involved in phosphorylation-dependent signaling. |
In summary, protein phosphorylation is a critical regulatory mechanism in cellular processes, and structural biology has provided essential insights into its molecular underpinnings. By studying how phosphorylation changes a protein's structure and function, scientists have gained valuable insights into cellular regulation and signal transduction.
Creative Biostructure offers specialized services in protein phosphorylation analysis and protein structure assay services, providing precise insights into phosphorylation sites, protein interactions, and signal transduction pathways. With state-of-the-art technologies and expert support, we deliver data that enhances your research outcomes. Partner with us to explore how our phosphorylation analysis services can drive your projects forward—contact us today to learn more.
References
- De Carvalho RSA, Rasel MSI, Khandelwal NK, Tomasiak TM. Cryo-EM reveals a phosphorylated R-domain envelops the NBD1 catalytic domain in an ABC transporter. Life Sci Alliance. 2024;7(11):e202402779.
- Humphrey SJ, James DE, Mann M. Protein phosphorylation: a major switch mechanism for metabolic regulation. Trends in Endocrinology & Metabolism. 2015;26(12):676-687.
- Liu Y, Chance MR. Integrating phosphoproteomics in systems biology. Computational and Structural Biotechnology Journal. 2014;10(17):90-97.
