Protein Aggregation Analysis
Protein aggregation is a crucial aspect to address in both scientific research and the biopharmaceutical industry. It can affect the stability, efficacy, and safety of protein-based products, making its analysis indispensable. At Creative Biostructure, we specialize in advanced protein structure analysis services, including comprehensive protein aggregation analysis. Explore with us the background of protein aggregation and the methods for its analysis!
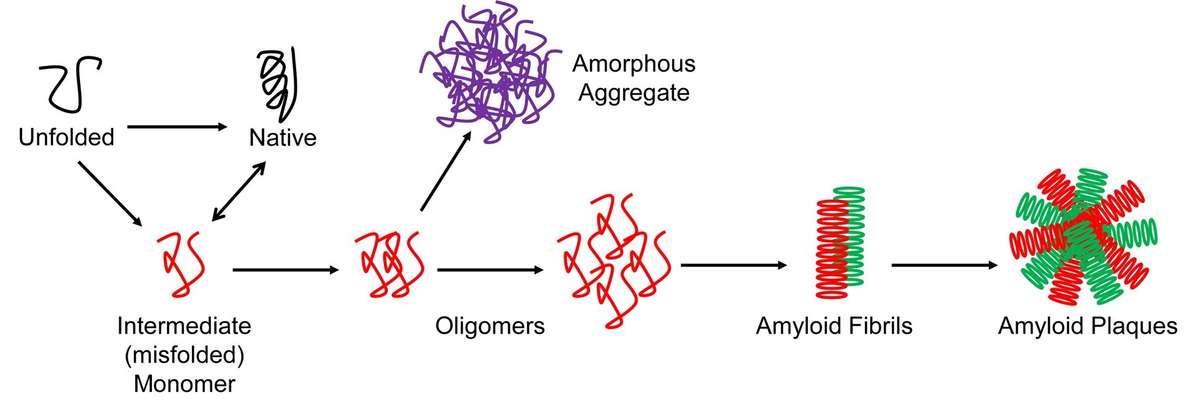 Figure 1. Protein aggregation. (Poothong et al., 2021)
Figure 1. Protein aggregation. (Poothong et al., 2021)
Background: Understanding Protein Aggregation
Proteins, the workhorses of cellular function, are intricately folded structures designed to perform precise biological roles. However, deviations from their native conformation often lead to aggregation, a phenomenon in which misfolded or denatured proteins self-associate into soluble oligomers or insoluble fibrils. Protein aggregation can disrupt normal cellular processes, trigger pathological conditions such as Alzheimer's disease, and compromise biopharmaceutical formulations. Understanding the nuances of protein aggregation is therefore essential for both basic research and practical applications.
Mechanisms of Protein Aggregation
The propensity of proteins to aggregate results from their delicate thermodynamic stability, which can be perturbed by various intrinsic and extrinsic factors.
Intrinsic Factors
- Primary Sequence and Conformational Flexibility: Proteins with regions of low sequence complexity or hydrophobic patches are predisposed to aggregation. These segments often evade proper folding or act as nucleation sites for aggregation.
- Post-Translational Modifications (PTMs): PTMs such as oxidation, glycation, or deamidation can destabilize native conformations, rendering proteins aggregation-prone.
Extrinsic Factors
- Environmental Stress: pH fluctuations, high ionic strength, temperature extremes, and mechanical agitation can unfold proteins, exposing hydrophobic cores that drive aggregation.
- Concentration Effects: High protein concentrations, often encountered in biopharmaceuticals, increase collision frequency, amplifying aggregation risk.
The aggregation process involves a cascade of molecular events, beginning with misfolding, followed by nucleation, and culminating in the growth phase where oligomers extend into fibrils. This complex interplay highlights the need for sophisticated analytical approaches.
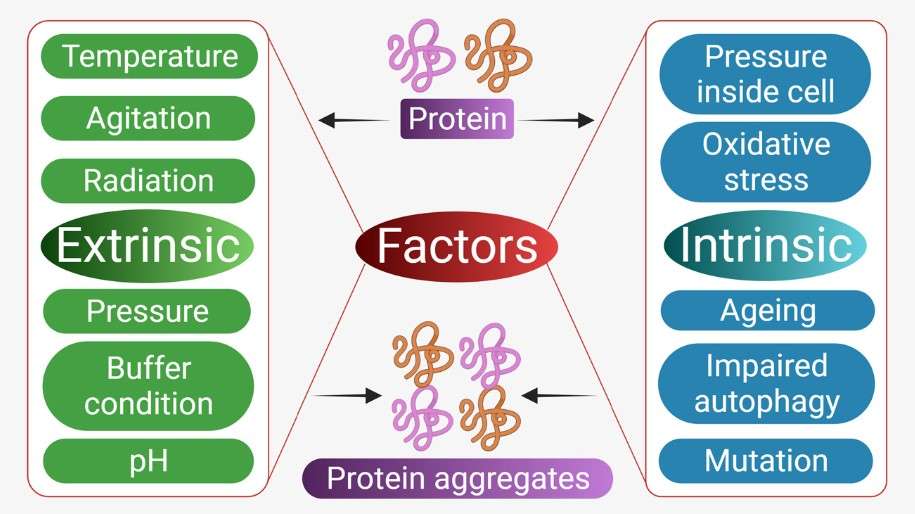 Figure 2. Factors affecting protein aggregations. (Bansal et al., 2021)
Figure 2. Factors affecting protein aggregations. (Bansal et al., 2021)
Analysis Methods for Protein Aggregation
The analysis of protein aggregation requires advanced methods to accurately detect, characterize, and quantify aggregates. These techniques, which include a range of physical, chemical and imaging methods, provide a multi-dimensional understanding of protein aggregation.
Light Scattering Techniques
Light scattering methods are essential for studying protein aggregates due to their non-invasive nature and ability to handle varying sample conditions.
- Dynamic Light Scattering (DLS): This technique measures fluctuations in scattered light intensity caused by particle motion in solution. It determines the hydrodynamic radius, size distribution, and polydispersity index of particles, making it ideal for identifying sub-micron aggregates. DLS excels in rapid and reproducible analysis of both soluble and insoluble aggregates in complex solutions.
- Static Light Scattering (SLS): Unlike DLS, SLS provides molecular weight and aggregation state measurements by analyzing the intensity of scattered light at different angles. It is especially valuable for quantifying oligomeric states and higher-order aggregates.
Spectroscopic Methods
Spectroscopy offers high sensitivity in detecting structural and compositional changes during aggregation.
- Fluorescence Spectroscopy: This method leverages fluorescence probes like Thioflavin T and Congo Red, which bind to amyloid fibrils, causing a distinct spectral shift. It is widely used to study amyloidogenic pathways and aggregate kinetics.
- Fourier Transform Infrared (FTIR) Spectroscopy: FTIR detects changes in protein secondary structure by analyzing characteristic amide bond vibrations. It provides detailed information about β-sheet content, often a hallmark of protein aggregation.
Microscopy-Based Approaches
Microscopy techniques offer direct visualization of aggregates, enabling qualitative and quantitative insights.
- Transmission Electron Microscopy (TEM): TEM delivers nanometer-scale resolution, revealing aggregate morphology and fibril architecture. It is particularly useful for studying amyloid fibrils and other fine structural details of aggregated proteins.
- Atomic Force Microscopy (AFM): AFM provides a 3D topographical view of protein aggregates, offering details on aggregate height, surface roughness, and mechanical properties. It is suitable for studying aggregation on surfaces and in solution.
Chromatographic Techniques
Chromatography remains a cornerstone in separating and characterizing protein aggregates based on size, charge, or other physicochemical properties.
- Size-Exclusion Chromatography (SEC): This technique separates aggregates from monomers and smaller oligomers based on hydrodynamic size. SEC is widely adopted for aggregation analysis in biopharmaceutical formulations, offering high-resolution results.
- Ion-Exchange Chromatography: Aggregates often differ in charge due to surface modifications. Ion-exchange chromatography separates aggregates and provides insights into charge heterogeneity.
Mass Spectrometry (MS)
Mass spectrometry excels in characterizing aggregate composition and post-translational modifications. Advanced MS techniques such as MALDI-TOF and ESI-MS detect small changes in mass that arise during aggregation. MS is particularly useful in identifying chemical modifications, such as oxidation or glycation, which may promote aggregation.
Emerging Technologies
As protein aggregation analysis evolves, new methods are continually developed to improve sensitivity, accuracy, and applicability.
- Microfluidics: This cutting-edge technology enables real-time monitoring of aggregation using minimal sample volumes. It integrates with optical detection systems for kinetic studies, making it ideal for high-throughput screening.
- Cryo-Electron Microscopy (Cryo-EM): Cryo-EM has revolutionized structural biology by capturing high-resolution images of aggregates in their native, hydrated state. It is invaluable for resolving aggregate structures that were previously challenging to study.
Applications of Protein Aggregation Analysis
Biopharmaceutical Development
Protein aggregation analysis ensures the stability and efficacy of therapeutic proteins such as monoclonal antibodies. It guides formulation optimization and safeguards against immunogenic responses caused by aggregates.
Disease Research
Aggregated proteins are implicated in neurodegenerative diseases, amyloidosis, and certain cancers. Detailed aggregation studies enhance our understanding of disease mechanisms and aid in developing diagnostic tools.
Quality Control in Manufacturing
Routine aggregation monitoring is critical for maintaining product consistency and compliance with regulatory standards in protein-based formulations.
Enzyme Engineering
Industries leveraging enzymes for biotechnological applications benefit from aggregation analysis to develop robust, aggregation-resistant proteins.
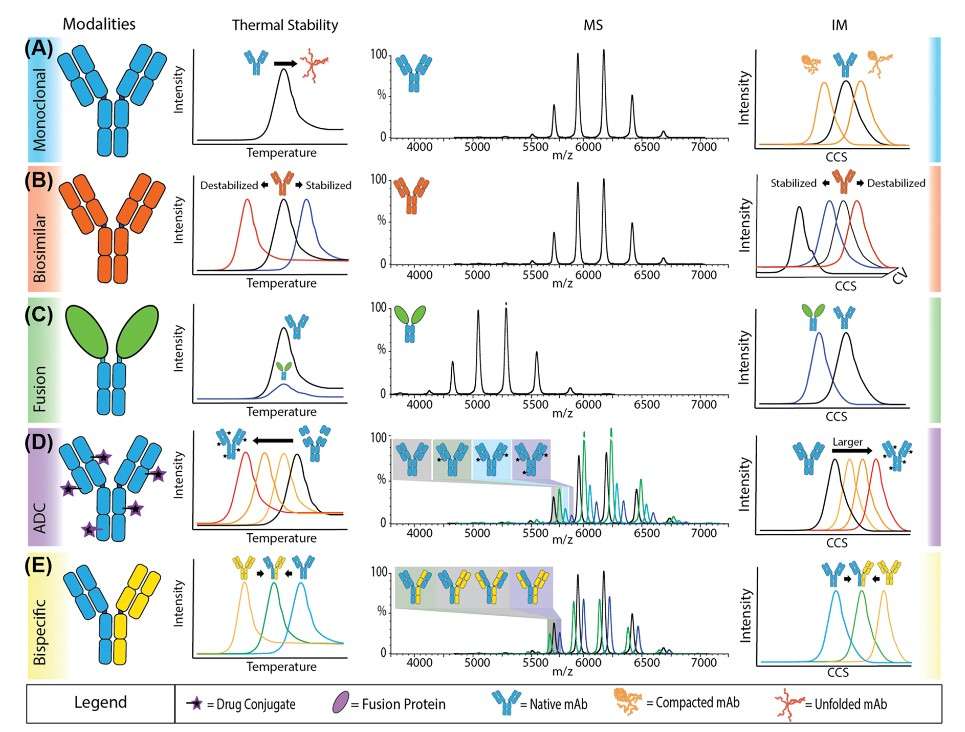 Figure 3. MS-based datasets for stability and aggregation analysis of biotherapeutic modalities Thermal protein-profiling experiments report shifts to lower Tm values, indicating a decrease in stability while higher values indicate an increase in stability. Changes in mass measurements can indicate different covalent structures or stoichiometries. Changes to CCS values recorded by IM indicate either more compact structures or larger, often unfolded structures. By applying gas-phase activation energy and monitoring unfolding, relative stability can be observed. (Vallejo et al., 2022)
Figure 3. MS-based datasets for stability and aggregation analysis of biotherapeutic modalities Thermal protein-profiling experiments report shifts to lower Tm values, indicating a decrease in stability while higher values indicate an increase in stability. Changes in mass measurements can indicate different covalent structures or stoichiometries. Changes to CCS values recorded by IM indicate either more compact structures or larger, often unfolded structures. By applying gas-phase activation energy and monitoring unfolding, relative stability can be observed. (Vallejo et al., 2022)
At Creative Biostructure, we offer cutting-edge protein aggregation analysis services. Our team of experts employs state-of-the-art technologies to ensure precise detection and characterization of protein aggregates. Contact us today to learn how our protein aggregation analysis services can support your research and development needs.
References
- Bansal R, Jha SK, Jha NK. Size-based degradation of therapeutic proteins - mechanisms, modelling and control. Biomolecular Concepts. 2021;12(1):68-84.
- Poothong J, Jang I, Kaufman RJ. Defects in protein folding and/or quality control cause protein aggregation in the endoplasmic reticulum. In: Agellon LB, Michalak M, eds. Cellular Biology of the Endoplasmic Reticulum. Vol 59. Springer International Publishing; 2021:115-143.
- Pukala TL. Mass spectrometric insights into protein aggregation. Britt H, Beveridge R, Calabrese A, eds. Essays in Biochemistry. 2023;67(2):243-253.
- Vallejo DD, Rojas Ramírez C, Parson KF, Han Y, Gadkari VV, Ruotolo BT. Mass spectrometry methods for measuring protein stability. Chem Rev. 2022;122(8):7690-7719.