MemPro™ stearoyl -CoA desaturase 1
Creative Biostructure provides custom MemPro™ gene-to-structure services for stearoyl -CoA desaturase 1.
Stearoyl-coenzyme A desaturase (SCD) is a δ-9 fatty acid desaturase that catalyzes the synthesis of monounsaturated fatty acids and has emerged as a key regulator of metabolism. Despite the abundance of monounsaturated fatty acids (MUFAs) in dietary fats, MUFAs are also synthesized de novo from saturated fatty acids by SCD enzymes. The major products of SCD, palmitoleic acid and oleic acid, are key substrates for the formation of complex lipids such as phospholipids, triglycerides, cholesterol esters, wax esters, and alkyl-2,3-diacylglycerols.
There are four isoforms (SCD1-4) in mouse while two have been found in human (hSCD1, hSCD5). SCD1 is highest in adipose and liver, but SCD5 is most abundant in brain and pancreas.
SCD1 folds into a mushroom-like architecture consisting of a stem of four transmembrane α-helices (TM stem) and a cytoplasmic domain. In the hSCD1 structure, two metal-binding sites are identified at the center of the cytoplasmic cap domain. A reduced iron center is determined as the substrate binding site to bind stearoyl CoA. The hSCD1 crystal structure provides a foundation for studying the determinants of regioselectivity and stereoselectivity in dehydrogenation by integral membrane desaturases.
SCD1 expression is highly regulated by a number of nutrients, hormones and environmental factors. Elevated SCD1 activity is correlated with increased plasma triglyceride levels, but this is highly dependent upon both genetic and dietary variables. SCD1 deficiency leads to lower plasma triglyceride levels under conditions that promote increased hepatic triglyceride synthesis and accumulation. Also, SCD1 deficiency affects plasma levels of HDL and LDL cholesterol. In humans and several animal models LDL cholesterol levels have been found to be increased by dietary saturated fatty acids, predominantly through modulation of LDL production and LDL clearance. The relation of SCD1 to LDL cholesterol balance may involve similar mechanisms by decreasing the ratio of MUFAs to saturated fatty acids.
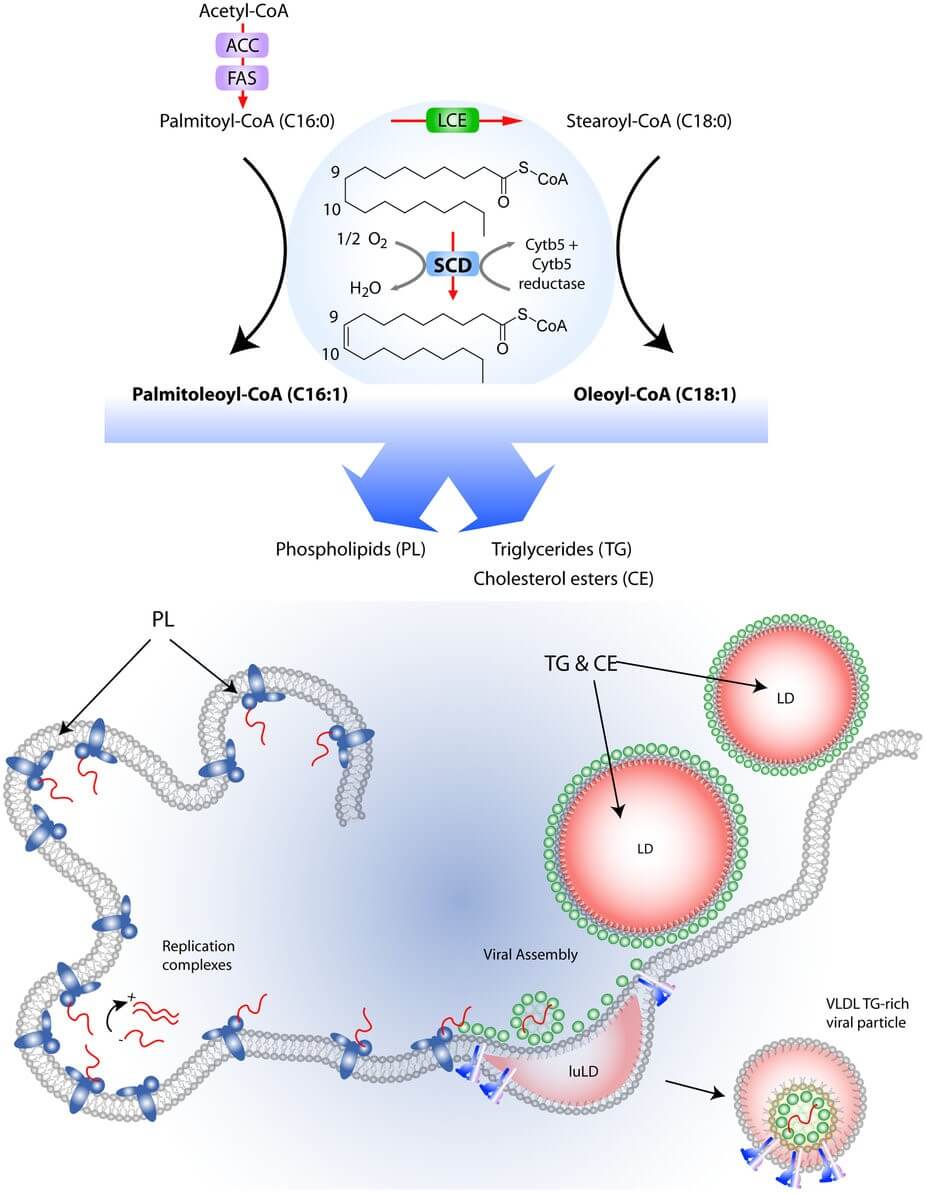 Figure 1. Role of SCD1 in regulating lipid metabolism
Figure 1. Role of SCD1 in regulating lipid metabolism
The dysregulation of SCD1 has been implicated in contributing to pathologies in diabetes, cancer and many metabolic syndromes. Cell-based and animal-tumor studies have shown that SCD1 plays a critical part in the regulation of lipid biosynthesis in cancer cells Furthermore, higher expression of SCD1 is associated with and higher plasma triglycerides and increased insulin levels. SCD1 serves a protective role to allow for continued MUFA synthesis and to maintain cellular lipid homeostasis. The conditionally essential nature of SCD1 may also explain why SCD1 inhibition reduces the survival of human tumor cells. Thus, SCD1 inhibitors may serve as therapeutic agents to treat cancers and metabolic diseases.
Reference
Wang H, Klein M G, Zou H, et al. Crystal structure of human stearoyl-coenzyme A desaturase in complex with substrate[J]. Nature structural & molecular biology, 2015, 22(7): 581-585.
Flowers M T, Ntambi J M. Role of stearoyl-coenzyme A desaturase in regulating lipid metabolism[J]. Current opinion in lipidology, 2008, 19(3): 248.