Comparison of Protein Expression Systems
Protein expression systems are central to biotechnology, enabling the production of recombinant proteins for a wide range of applications, from industrial enzymes and diagnostics to pharmaceuticals and academic research. At Creative Biostructure we offer protein expression services using various expression systems. Here we provide a detailed comparison of different protein expression systems, examining recombinant protein expression methods, factors influencing host system selection, and the characteristics of cell-free, bacterial, yeast, mammalian, and insect expression systems. Understanding the unique advantages and limitations of each will help you choose the optimal solution for your project.
Recombinant Protein Expression Methods
Recombinant protein expression involves the introduction of a gene of interest into a host system capable of transcribing and translating it into the desired protein. This process typically involves gene cloning, transformation/transfection, expression induction, protein folding and modification, and purification. For more information, please visit the basics of protein expression page.
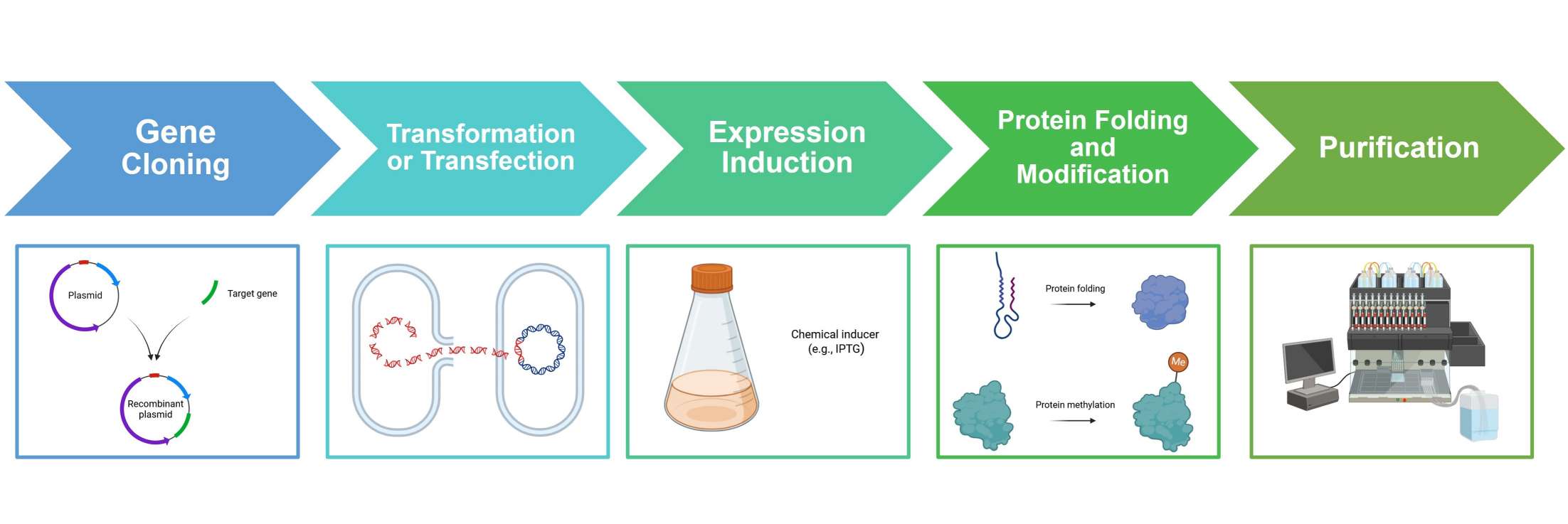 Figure. 1: Process of recombinant protein expression. (Created with BioRender.com)
Figure. 1: Process of recombinant protein expression. (Created with BioRender.com)
Critical Parameters to Select a Host System
Selecting a host system for protein expression is a multifaceted decision influenced by several critical parameters:
- Protein Complexity: The structural and functional complexity of the target protein dictates the need for advanced PTMs, proper folding, and multi-subunit assembly. For simple proteins, bacterial systems may suffice, while complex proteins require eukaryotic hosts.
- Post-Translational Modifications: PTMs such as glycosylation, phosphorylation, acetylation, and disulfide bond formation are critical for many eukaryotic proteins. Bacterial systems lack these capabilities, whereas mammalian systems provide the most authentic PTMs.
- Yield Requirements: High yield is crucial for industrial applications. Bacterial and yeast systems excel at producing large quantities of protein, often at the expense of PTM fidelity.
- Protein Stability and Activity: Host systems differ in their ability to produce stable, bioactive proteins. Authentic folding and modifications are essential for therapeutic and diagnostic proteins.
- Production Speed: Time constraints favor fast-growing systems such as bacteria and yeast, while mammalian and insect systems typically require longer development cycles.
- Scalability: Industrial-scale protein production requires host systems that are easily scalable. Bacteria and yeast systems offer easy scalability.
- Cost Constraints: Budget limitations often dictate the choice of a cost-effective host system, with bacterial systems being the least expensive and mammalian systems being the most resource intensive.
- Protein Toxicity: Proteins that are toxic to the host cell may require the use of specialized systems, such as cell-free expression or tightly controlled inducible systems.
Comparison of Protein Expression Systems
Recombinant protein expression technology enables a variety of downstream applications, including the analysis of gene regulation, protein structure and function, protein-protein interactions, and drug discovery and development. Using the right protein expression system for your specific application can be critical to your success. The following table summarizes the benefits and challenges of each protein expression system:
| Host System | Advantages | Challenges |
| Bacterial |
|
|
| Mammalian |
|
|
| Yeast |
|
|
| Insect |
|
|
| Cell-Free |
|
|
In a review of guidelines for selecting appropriate gene expression systems for recombinant protein production, the most commonly used and accessible systems were visually scored according to 7 important parameters.
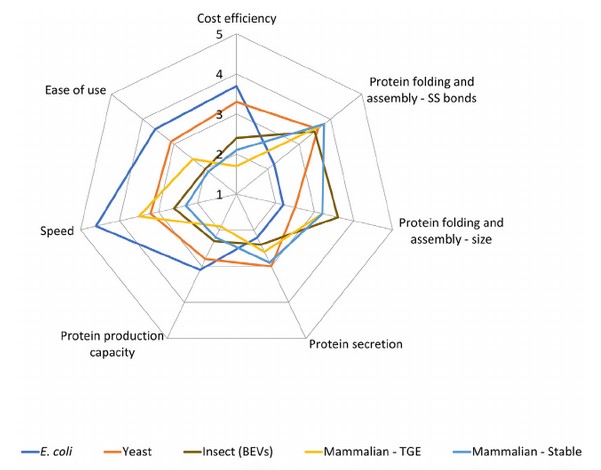 Figure. 2: Comparative overview of the characteristics associated with the major gene expression systems. Scoring: Ease of use: (1) Possible with SOP + user training + 1 year hands-on experience; (2) possible with SOP + user training + short-term experience; (3) possible with SOP + user training; (4) possible with SOP+ short-term experience; (5) possible with SOP only. Speed: (1) >8 weeks; (2) 4-8 weeks; (3) 1-4 weeks; (4) 3-7 days; (5) 1-3 days. Protein production capacity: (1) <1 mg/L; (2) 1-5 mg; (3) 5-20 mg/L; (4) 20-100 mg/L; (5) >100 mg/L. Protein secretion: (1) <1 mg/L; (2) 1-5 mg/L; (3) 5-20 mg/L; (4) 20-100 mg/L; (5) >100 mg/L. Protein folding and assembly-size: (1) <50 kDa; (2) 50-100 kDa; (3) 100-250 kDa; (4) 250-500kDa; (5) >500 kDa. Protein folding and assembly—SS bonds: (1) 1 SS bond; (2) 2 SS bonds; (3) 3-4 SS bonds; (4) 5-10 SS bonds; (5) >10 SS bonds. Cost efficiency: (5) <50 €/L; (4) 50-100 €/L; (3) 100-500 €L; (2) 500-1,000 €ЛL; (1) >1,000 €/L. (Schütz A et al., 2023)
Figure. 2: Comparative overview of the characteristics associated with the major gene expression systems. Scoring: Ease of use: (1) Possible with SOP + user training + 1 year hands-on experience; (2) possible with SOP + user training + short-term experience; (3) possible with SOP + user training; (4) possible with SOP+ short-term experience; (5) possible with SOP only. Speed: (1) >8 weeks; (2) 4-8 weeks; (3) 1-4 weeks; (4) 3-7 days; (5) 1-3 days. Protein production capacity: (1) <1 mg/L; (2) 1-5 mg; (3) 5-20 mg/L; (4) 20-100 mg/L; (5) >100 mg/L. Protein secretion: (1) <1 mg/L; (2) 1-5 mg/L; (3) 5-20 mg/L; (4) 20-100 mg/L; (5) >100 mg/L. Protein folding and assembly-size: (1) <50 kDa; (2) 50-100 kDa; (3) 100-250 kDa; (4) 250-500kDa; (5) >500 kDa. Protein folding and assembly—SS bonds: (1) 1 SS bond; (2) 2 SS bonds; (3) 3-4 SS bonds; (4) 5-10 SS bonds; (5) >10 SS bonds. Cost efficiency: (5) <50 €/L; (4) 50-100 €/L; (3) 100-500 €L; (2) 500-1,000 €ЛL; (1) >1,000 €/L. (Schütz A et al., 2023)
Protein expression systems are essential tools tailored to meet the diverse needs of research and industry. Creative Biostructure offers customized solutions for all major expression systems—bacterial, yeast, mammalian, insect and cell-free—to ensure that your research or industrial goals are achieved with precision and efficiency. Contact us today and improve your protein expression capabilities!
Reference
- Schütz A, Bernhard F, Berrow N, et al. A concise guide to choosing suitable gene expression systems for recombinant protein production. STAR Protocols. 2023;4(4):102572.