Yeast Expression
Recombinant protein production is a cornerstone of modern biotechnology and a multibillion-dollar industry. It underpins the manufacturing of therapeutic proteins, enzymes, vaccines, and research tools, driving advancements in medicine, agriculture, and industrial processes. Among the several expression systems available, yeast cell factories have emerged as a versatile and robust platform. Yeast systems uniquely combine the simplicity and cost-effectiveness of microbial platforms like Escherichia coli with key eukaryotic traits, such as complex protein folding, secretion pathways, and post-translational modifications (PTMs). These features make yeast expression systems a central tool for the production of high-quality recombinant proteins for various applications.
At Creative Biostructure, we offer protein expression services using advanced yeast expression systems. Read on to discover how we can take advantage of the yeast expression system.
Applications of Yeast Protein Expression Systems
The versatility of yeast systems has opened up numerous applications in various fields. These applications take advantage of yeast's ability to produce diverse proteins with high efficiency and quality.
Biocatalysis
Yeast cells are widely used as biocatalysts due to their ability to express enzymes and other catalytic proteins. These biocatalysts find applications in chemical synthesis, environmental remediation, and biofuel production.
Combinatorial Library Presentation
Yeast surface display technology allows the presentation of large combinatorial libraries of proteins or peptides. This technique is invaluable for antibody discovery, protein engineering, and drug discovery.
Whole-Cell Vaccines
Yeast systems have been harnessed to express antigens on their surface, creating whole-cell vaccines. These vaccines are cost-effective to produce and offer a promising approach to combating infectious diseases, particularly in low-resource settings.
Anticancer drug discovery
The yeast expression system plays an important role in anticancer drug discovery by serving as a simplified and cost-effective model to study cellular processes that are conserved in mammalian cells. In particular, Saccharomyces cerevisiae shares a high degree of conservation in gene sequences, protein functions and cellular pathways with humans, making it an ideal surrogate for understanding cancer-related mechanisms.
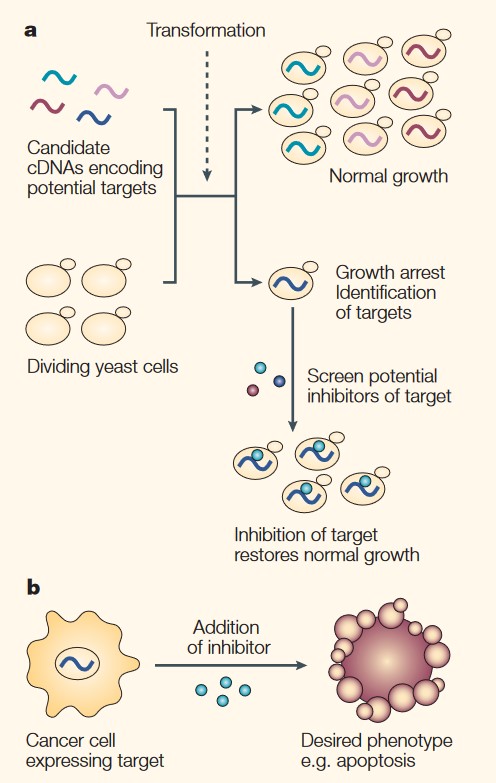 Figure 1. Target-based screens for anticancer drugs using yeast. a | Expression of human proteins in yeast can lead to an arrest in cell growth and division, and this can be used as a basis for drug screens. Candidate cDNAs that encode potential targets—that is, proteins that are upregulated or aberrantly activated in human cancers—are transformed into yeast. Those that block growth are identified and can be used in small-molecule screens for agents that restore growth to the engineered strain and are therefore inhibitors of the human proteins. b | The identification of inhibitors from yeast target-based screens can be validated in mammalian cells that express the target gene. The inhibitor is tested for its ability to induce a desired phenotype, such as apoptosis, indicating its potential for use as an anticancer agent. (Simon JA and Bedalov A, 2004)
Figure 1. Target-based screens for anticancer drugs using yeast. a | Expression of human proteins in yeast can lead to an arrest in cell growth and division, and this can be used as a basis for drug screens. Candidate cDNAs that encode potential targets—that is, proteins that are upregulated or aberrantly activated in human cancers—are transformed into yeast. Those that block growth are identified and can be used in small-molecule screens for agents that restore growth to the engineered strain and are therefore inhibitors of the human proteins. b | The identification of inhibitors from yeast target-based screens can be validated in mammalian cells that express the target gene. The inhibitor is tested for its ability to induce a desired phenotype, such as apoptosis, indicating its potential for use as an anticancer agent. (Simon JA and Bedalov A, 2004)
Advantages and Limitations of Yeast Expression Systems
| Advantages of Yeast Expression Systems | |
| Faster, Easier, and Less Expensive Growth | Yeast cells grow rapidly in inexpensive media, making them cost-effective compared to mammalian systems. They can thrive in simple culture conditions without requiring complex or expensive growth factors, significantly reducing production costs. Additionally, yeast can grow to high cell densities in bioreactors, further enhancing productivity. |
| High Levels of Protein Expression | Yeast systems can achieve protein expression levels 10-100 times higher than E. coli. This high yield is due to their strong promoters, efficient transcription and translation machinery, and ability to continuously produce proteins under optimized conditions. These characteristics make yeast ideal for industrial-scale production. |
| Secretion of Proteins | Yeast cells have a well-developed secretory pathway that allows the production of secreted proteins. The expression of recombinant proteins as secretory products simplifies downstream processing and purification because the proteins can be harvested directly from the culture medium. This reduces the complexity and cost of purification steps. |
| Post-Translational Modifications (PTMs) | Unlike E. coli, yeast cells can perform essential PTMs, such as glycosylation, phosphorylation, and disulfide bond formation. These modifications are critical for the functionality, stability, and therapeutic efficacy of many human proteins. Moreover, advances in glycoengineering have enabled yeast systems to produce proteins with human-like glycosylation patterns, addressing a key limitation of earlier systems. |
| Robust Cellular Structure | Yeast cells have a robust cellular structure that allows them to withstand environmental stress and thrive under industrial fermentation conditions. Their resilience makes them well suited for large-scale production processes, including high-stress environments such as high cell density fermentation. |
| Synthesis of Complex and Multisubunit Proteins | Yeast cells can efficiently express complex proteins with multiple subunits, including those requiring accurate folding and assembly. Their eukaryotic machinery ensures that the structural integrity and biological activity of these proteins are preserved. |
| Industrial Fermentation | Yeast cells are compatible with fermentation processes that are scalable and efficient for industrial applications. The use of well-established bioreactors for yeast culture enables large-scale production of recombinant proteins with high reproducibility and productivity. |
| Limitations of Yeast Expression Systems | |
| Limited Choice of Vectors | Compared to bacterial systems such as E. coli, yeast systems have fewer vectors available for genetic manipulation. This limitation can limit the flexibility of cloning strategies and expression optimization. |
| Unresolved Biological Activity | In some cases, recombinant proteins expressed in yeast systems exhibit reduced or unresolved biological activity. This problem is often due to improper folding, incomplete PTMs, or aberrant glycosylation patterns. Efforts are underway to improve protein quality through strain engineering and optimization of culture conditions. |
| Proteolytic Degradation | Yeast cells have active proteases that can degrade foreign proteins, reducing yields and complicating production. To mitigate this problem, protease-deficient yeast strains have been developed. These strains are engineered to lack specific protease genes, thereby improving protein stability and yield. |
| Glycosylation Pattern Differences | While yeast cells can perform glycosylation, their glycan structures differ from those of humans. For example, yeast tends to add hypermannosylation, which can trigger immune responses in therapeutic applications. Glycoengineering has made significant progress in humanizing yeast glycosylation pathways, but achieving complete mimicry remains a challenge. |
Unlock the full potential of your research and development with our comprehensive yeast protein expression services. Whether you need rapid, cost-effective protein production or customized solutions for complex and multi-subunit proteins, our expertise ensures reliable results tailored to your needs. Contact us today to discuss your project and discover how our yeast expression systems can help you succeed!
References
- Baghban R, Farajnia S, Rajabibazl M, et al. Yeast expression systems: overview and recent advances. Mol Biotechnol. 2019;61(5):365-384.
- Böer E, Steinborn G, Kunze G, Gellissen G. Yeast expression platforms. Appl Microbiol Biotechnol. 2007;77(3):513-523.
- Çelik E, Çalık P. Production of recombinant proteins by yeast cells. Biotechnology Advances. 2012;30(5):1108-1118.
- Schreuder MP, Mooren ATA, Toschka HY, Theo Verrips C, Klis FM. Immobilizing proteins on the surface of yeast cells. Trends in Biotechnology. 1996;14(4):115-120.
- Simon JA, Bedalov A. Yeast as a model system for anticancer drug discovery. Nat Rev Cancer. 2004;4(6):481-487.